Quiz-1
advertisement

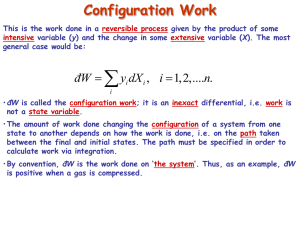
ECHE 311-SPRING 2015 Quiz 1 1. The general first-law equation for a mechanically reversible, nonflow process is given as: d (nU ) dQ Pd (nV ) (1) If in addition the process occurs at constant volume, then the work is zero, and dQ d (nU ) Integration yields Q nU Show that for mechanically reversible, constant-pressure, nonflow process, the transferred equals to the enthalpy change of the system. 25 points heat 2. Define the state function in thermodynamics. Does a thermodynamic state reflects changes at the molecular (microscopic level). Define the first law of thermodynamics. The first law applies to a. the system and surroundings b. the system alone define and identify the extensive properties define and identify the intensive properties 25 points 3. Define the differences between a constant temperature isothermal process and a reversible adiabatic process 25 points 4. Explain the iteration procedure for calculation of T when To and Q or H are given in the specific heat capacity equation. Hint: Read the Chap “ Heat Effects” in your text book and my handouts. 25 points