Lecture 24
advertisement

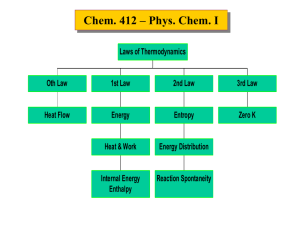
Thermochemistry (4.1-4.3) • Enthalpy (ΔH) is related to the heat exchange that occurs during a chemical or physical process under constant pressure – Processes that generate heat are exothermic, processes that absorb heat are endothermic – Phase changes also have enthalpies associated with them (e.g., heat of vaporization) – Enthalpies are state functions • Heats of reaction (ΔHrxn) can be measured or calculated for most reactions – They can be measured through calorimetry (e.g., bomb or solution calorimetry) – They can be calculated using Hess’s Law • Heats of formation (ΔHf) are the heats associated with forming one mole of a compound from its elements in their standard states – Heats of formation are typically reported under standard conditions: 298.15 K and a pressure of 1 bar for each gaseous component – Why would this be a problem in biochemistry? Temperature Dependence of Enthalpy (4.4) • Heat capacities (CP or CV) are related to the amount of heat needed to raise the temperature of a substance by 1 °C (section 2.4) – This quantity tells us how much heat a substance can store (e.g., water has a high heat capacity thus it stores lots of heat, metals have low heat capacities) – Over short temperature ranges, the heat capacities are typically temperature independent (i.e., constant value) • The amount of heat evolved or absorbed during a chemical process at a given temperature is related to a number of factors – The standard heats of formation are useful, but only tell part of the story – If the reaction occurs at elevated (or depressed) temperatures, heat capacities of the reactants and products are needed – If phase changes occur, then enthalpies associated with the phase changes are needed H 0 rxn,T H 0 rxn,298K T C T'dT' P 298K prod react i j CP T' iCP,i T' j CP, j T' Differential Scanning Calorimetry (4.6) • Differential scanning calorimetry (DSC) is a way of measuring energy changes associated with physical transitions – Phase transitions in compounds, metals, polymers – Denaturation of biopolymers can be considered a phase transition • DSC uses differences in heating of a sample and a standard to determine thermodynamic parameters – Sample and standard temperatures are ramped up over time, but are kept equal – The difference in the amount of heat needed to maintain temp. is the difference in heat capacities (assuming no chemical or physical changes) • When a chemical or physical change occurs, the heating of the sample changes drastically – During a phase change, heat goes towards changing physical state, not temp. increase – For protein denaturation, the heat capacity of the natural protein (CN) is significantly different than the denatured protein (CD) – The melting temp. of the protein is the temp. at the peak maximum – The area under the curve is the enthalpy associated with the corresponding transition Enthalpy as a State Function Bomb Calorimetry Solution Calorimetry Differential Scanning Calorimeter Phase Change in DSC Protein Denaturation in DSC