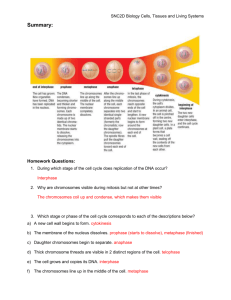
Lab 8 - Cell Reproduction: Cell Division and the Cell Cycle A living
advertisement

Lab 8 - Cell Reproduction: Cell Division and the Cell Cycle A living cell has the capacity to divide unless that capacity is lost during differentiation and maturation. Of primary concern in a dividing cell is the orderly apportionment of the hereditary information contained within the nucleus. This information is organized into large structures called chromosomes. Cell division is the orderly and predictable partitioning of cellular material from the parental cell to the daughter cells. The term mitosis specifically refers to the partitioning of the chromosomes. The time it takes for a single parental cell to divide, thus forming two daughter cells is called the cell cycle. Three major stages have been identified as part of the cell cycle. Some of these have been further subdivided. These stages are briefly described below: Interphase Gap 1 (G1) Phase - the cell undergoes general growth, increase in numbers of organelles, and an increase in the volume of cytoplasm. Synthesis (S) Phase - the chromosomes are duplicated. Gap 2 (G2) Phase - the cell makes special structures (centrioles, spindle fibers, etc.) that will be needed for division. Mitosis Prophase and prometaphase nucleolus disappears chromosomes condense and become visible as rod-shaped structures mitotic spindle begins to form nuclear membrane separates into fragments spindle microtubules invade nucleus and attach to chromosomes chromosomes move towards an imaginary equatorial plane at the center of the cell Metaphase duplicated chromosomes line up at equatorial plane Spindle fibers attach at the centromeres of the chromosomes Anaphase Duplicates uncouple at the centromere Spindle fibers shorten One complete set of unduplicated chromosomes moves towards each of the poles Telophase Unduplicated chromosomes arrive at the poles Nuclear membrane reforms Nucleolus reforms Chromosomes stretch out into invisible threads Cytokinesis - this process involves the division of the cytoplasm and its contents by the formation of new cytoplasmic membrane and/or cell walls. It usually starts in late anaphase or in telophase and is completed either in late telophase or in G1 of the next interphase. 1 Biologists define growth as an irreversible increase in size. Thus, growth could be accomplished in two ways: 1) by increasing the number of cells in a region and/or 2) by existing cells increasing in volume/size. In higher animals, such as ourselves, growth is largely by cell division or the addition of new cells to a tissue (from some other area in the body). In plants, however, growth occurs by cell division and cell enlargement. Consider a plant root. The increase in the length of a root is mediated largely by a root meristem located at the tip of the root, just behind the root cap (see Fig in textbook). Immediately behind the root meristem is the region of cell maturation. In this region, newly formed cells begin to differentiate (acquire specific structural and functional characteristics). For example, some of the cells in this region of the root develop into root hairs and vascular tissue. In this lab exercise you will use prepared slides and the compound light microscope to study the nature of growth in a typical root. In order to successfully complete this study an understanding of the cell cycle and mitosis is necessary. You will begin by learning to recognize cells in interphase and mitosis (non-dividing and dividing cells, respectively). You will also learn to recognize cells in the various stages of mitosis. Once confident in this assessment, you will determine the nature of growth in the root meristem and the region of cell maturation. In addition, you will determine which stage of the cell cycle and which stage of mitosis is of the longest duration. Remember, regions of cell division will be expected to have a higher mitotic index than a region where cells are not actively dividing. In addition, cells in all stages of the cell cycle should be evident in actively growing regions. Fig 5—Growing root tissue (from Biology, Campbell and Reece, 6th ed.) 2 A. Determination of the Mitotic Index The mitotic index is the ratio of dividing cells to the total number of cells in a population. Prepared slides of onion root tips can be used to measure this quantity. The slides provided to you are longitudinal sections showing the root cap (dead cells), the root meristem, and the region of cell maturation. Procedure: 1) Find and examine the root meristem, and the region of cell maturation using the high power objective lens. Be sure you can correctly identify these two regions. There are no labels within the longitudinal sections so if you are unsure ask your instructor. 2) Within the root meristem, identify at least one cell that is in each of the phases listed on worksheet #1. As you find each one, make a drawing in the space provided. Have your instructor check your cell in the microscope and initial your drawing. 3) Determine the mitotic index in the root meristem (use worksheet #2) as follows: a) Choose at least 100 cells in this region (one way to do this is to pick 2 or 3 columns of cells and count all cells in those columns that lie that region). b) Record in the worksheets the number of cells (of the hundred chosen) that are in interphase, prophase, metaphase, anaphase, and telophase/Cytokinesis (make sure you do not confuse prophase with interphase, or it will result in an abnormally high mitotic index). c) Calculate the mitotic index: Mitotic index = # cells dividing/total # cells observed # cells dividing = #prophase + #metaphase + #anaphase + #telophase/cytokinesis 4) Repeat step 3 for the region of cell maturation (use worksheet #3). 5) Perform a Chi-Square contingency test with the data obtained to determine if the mitotic indexes of the two regions examined are significantly different. Instructions for performing this statistical test are located in Part D. 3 Title: _____________________________________________________ Date: ___________ Phase of Cell Cycle Drawing Interphase Prophase Or Prometaphase Metaphase Anaphase Telophase Cytokinesis 4 Title: ____________________________________________(Meristem) Date: ___________ Phase # Cells Percent Interphase Prophase and prometaphase (*) Metaphase (*) Anaphase (*) Telophase/Cytokinesis (*) # Cells Dividing (*) Mitotic Index = ---------------------------------------- = _____________ Total # Cells Observed (**) Title: _________________________________________(Zone of Maturation) Date: ___________ Phase # Cells Percent Interphase Prophase and prometaphase (*) Metaphase (*) Anaphase (*) Telophase/Cytokinesis (*) # Cells Dividing (*) Mitotic Index = ---------------------------------------- = _____________ Total # Cells Observed (**) * # Cells Dividing = #Prophase/prometaphase + #Metaphase + #Anaphase + #Telophase ** Total # Cells = # Cells Dividing + # Cells in Interphase 5 B. Statistical Analyses Chi-Square Contingency Analysis This statistical test is employed with count data that is grouped into a matrix defined by two variables. In this case, the two variables are 1) region of root examined (meristem and region of maturation) and 2) phase of the cell cycle (interphase and mitosis). Contingency analysis is simply a test of interaction between the two variables. The null hypothesis for the statistical analysis is that the effect of one variable is not influenced by the other. In this test, this means that the number of cells in interphase or mitosis is not contingent upon, or influenced by, the region of the root tip. 1) Transpose data from the previous worksheets to complete the following contingency table: Title: ______________________________________________________Date: ___________ Region of Root Phase Meristem Cell Maturation Interphase (a) (b) Mitosis (c) (d) Total (T) (a+b+c+d) 2) Calculate the Chi-Square value for the above data by using the following equation: Chi-Square = (ad – bc - 0.5T)2 (T) (a+b)(a+c)(b+d)(c+d) 3) Determine the degrees of freedom: df = (No. phases - 1) (No. of regions - 1) Note: the number of phases and regions referred to in the determination of df are those that are indicated in the table. 4) Compare the calculated Chi-Square value with a tabled critical value for the corresponding number of degrees of freedom and a confidence level of 95%. Use Table 1 to determine the critical value (page 61 of the lab manual). Calculated ChiSquare values greater than the table value (for given df and confidence level) indicate that differences are NOT likely due to chance alone (e.g. are statistically significantly different), and the null hypothesis is rejected. Thus, if your values have been determined to be significantly different, the mitotic indexes previously calculated can be compared. 6