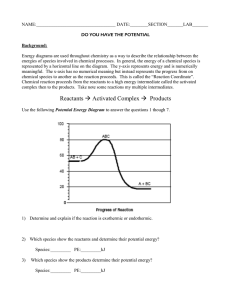
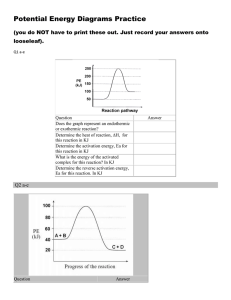
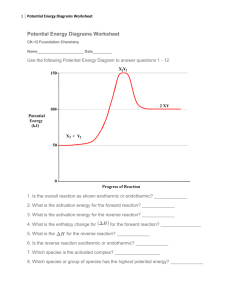
Kinetics & Potential Energy Diagrams Worksheet
advertisement
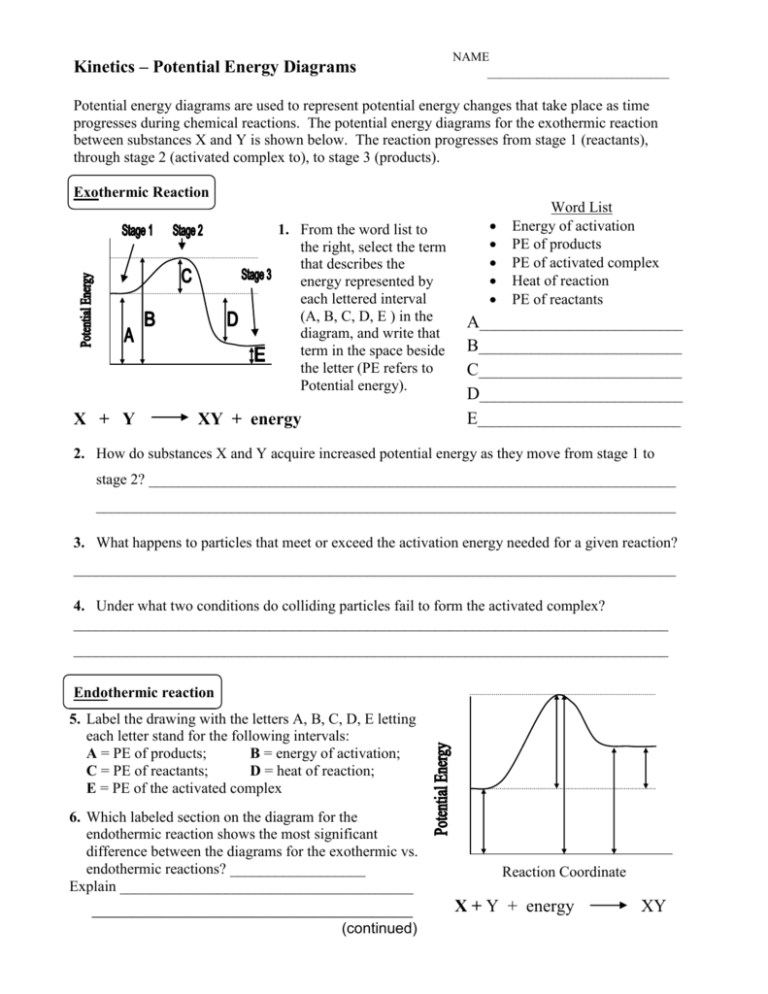
Kinetics – Potential Energy Diagrams NAME _____________________________ Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products). Exothermic Reaction 1. From the word list to the right, select the term that describes the energy represented by each lettered interval (A, B, C, D, E ) in the diagram, and write that term in the space beside the letter (PE refers to Potential energy). X + Y XY + energy Word List Energy of activation PE of products PE of activated complex Heat of reaction PE of reactants A___________________________ B___________________________ C___________________________ D___________________________ E___________________________ 2. How do substances X and Y acquire increased potential energy as they move from stage 1 to stage 2? ______________________________________________________________________ _____________________________________________________________________________ 3. What happens to particles that meet or exceed the activation energy needed for a given reaction? ________________________________________________________________________________ 4. Under what two conditions do colliding particles fail to form the activated complex? _______________________________________________________________________________ _______________________________________________________________________________ Endothermic reaction 5. Label the drawing with the letters A, B, C, D, E letting each letter stand for the following intervals: A = PE of products; B = energy of activation; C = PE of reactants; D = heat of reaction; E = PE of the activated complex 6. Which labeled section on the diagram for the endothermic reaction shows the most significant difference between the diagrams for the exothermic vs. endothermic reactions? __________________ Explain _______________________________________ Exothermic reaction ________________________________ (continued) Reaction Coordinate X + Y + energy XY 7. On the diagram below, draw a dotted line to show the pathway of a catalyzed reaction. Add the label C' to show the interval that represents the activation energy for the catalyzed reaction. Reaction Coordinate 8. What is the name of the part of the diagram for the catalyzed pathway that is most changed from that on the diagram for the non-catalyzed pathway? _____________________________ Explain why it is changed. ________________________________________________________ ______________________________________________________________________________ 9. What affect does this change have on the rate of the reaction?_____________________________ 10. According to the collision theory, why does the presence of a catalyst increase the rate of a reaction?_______________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________