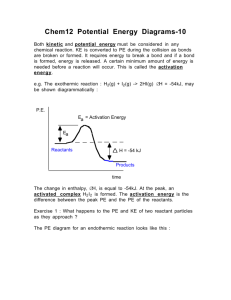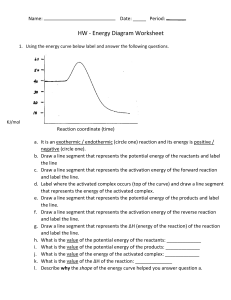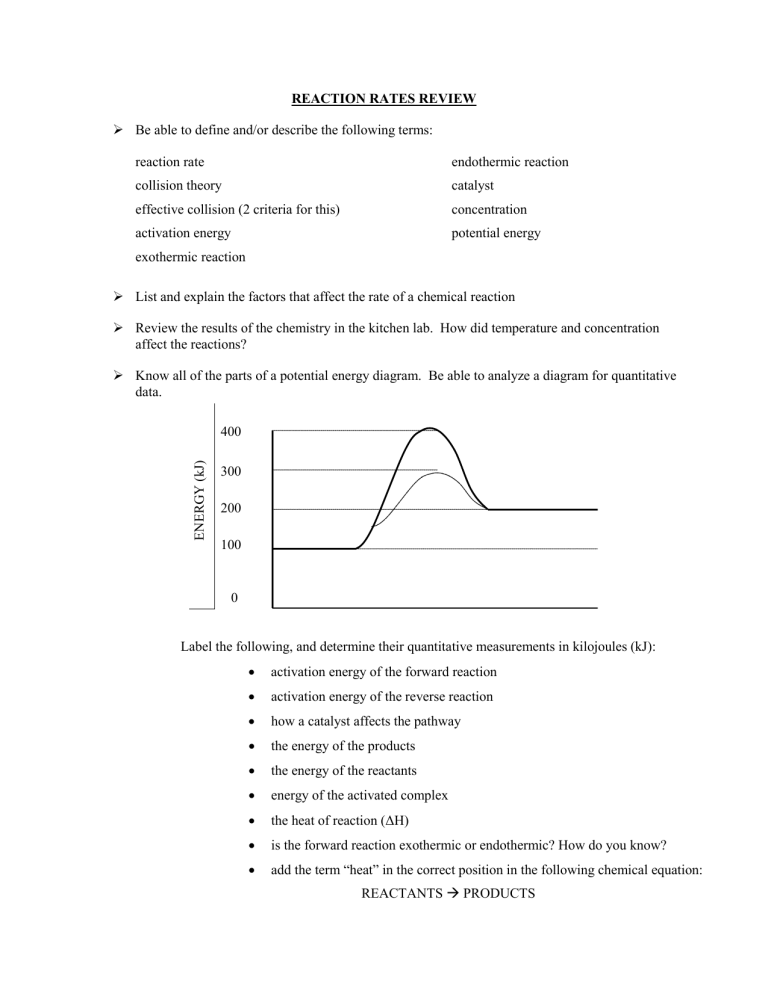
REACTION RATES REVIEW Be able to define and/or describe the following terms: reaction rate endothermic reaction collision theory catalyst effective collision (2 criteria for this) concentration activation energy potential energy exothermic reaction List and explain the factors that affect the rate of a chemical reaction Review the results of the chemistry in the kitchen lab. How did temperature and concentration affect the reactions? Know all of the parts of a potential energy diagram. Be able to analyze a diagram for quantitative data. ENERGY (kJ) 400 300 200 100 0 Label the following, and determine their quantitative measurements in kilojoules (kJ): • activation energy of the forward reaction • activation energy of the reverse reaction • how a catalyst affects the pathway • the energy of the products • the energy of the reactants • energy of the activated complex • the heat of reaction (ΔH) • is the forward reaction exothermic or endothermic? How do you know? • add the term “heat” in the correct position in the following chemical equation: REACTANTS PRODUCTS