Enthalpy - Mwiseman.com
advertisement
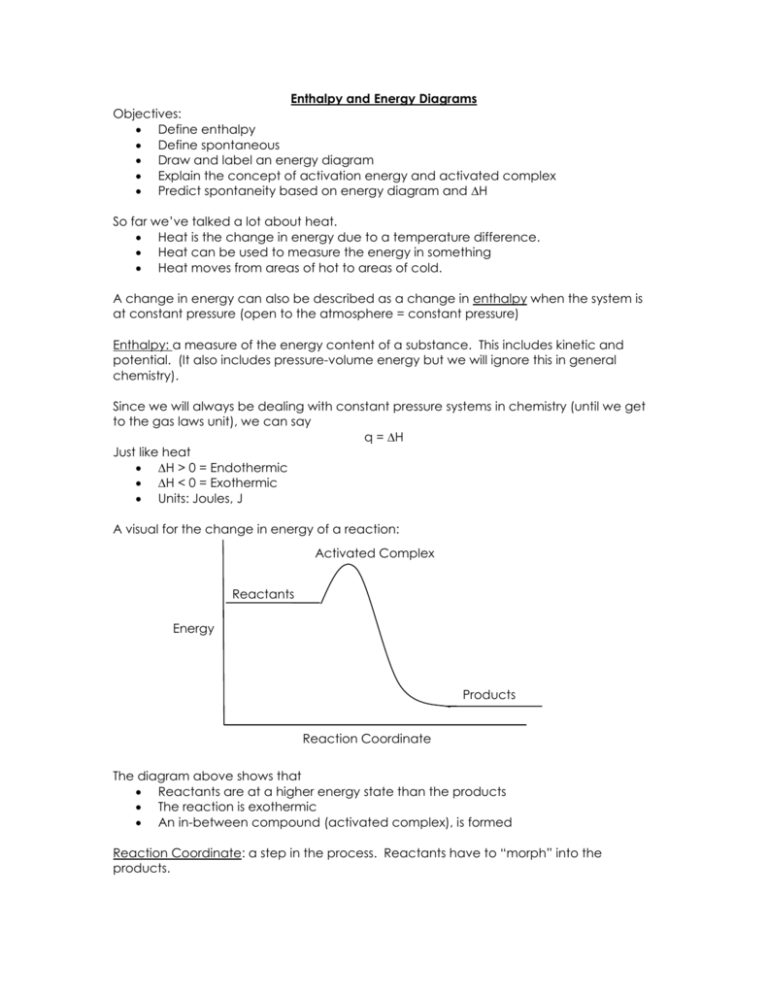
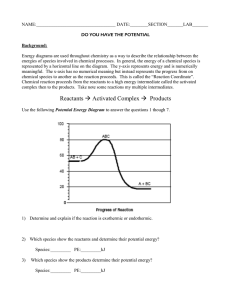
Enthalpy and Energy Diagrams Objectives: Define enthalpy Define spontaneous Draw and label an energy diagram Explain the concept of activation energy and activated complex Predict spontaneity based on energy diagram and H So far we’ve talked a lot about heat. Heat is the change in energy due to a temperature difference. Heat can be used to measure the energy in something Heat moves from areas of hot to areas of cold. A change in energy can also be described as a change in enthalpy when the system is at constant pressure (open to the atmosphere = constant pressure) Enthalpy: a measure of the energy content of a substance. This includes kinetic and potential. (It also includes pressure-volume energy but we will ignore this in general chemistry). Since we will always be dealing with constant pressure systems in chemistry (until we get to the gas laws unit), we can say q = H Just like heat H > 0 = Endothermic H < 0 = Exothermic Units: Joules, J A visual for the change in energy of a reaction: Activated Complex Reactants Energy Products Reaction Coordinate The diagram above shows that Reactants are at a higher energy state than the products The reaction is exothermic An in-between compound (activated complex), is formed Reaction Coordinate: a step in the process. Reactants have to “morph” into the products. Reaction Mechanism: how the substances “morph” into products, the steps involved in changing from reactant to product Animations of reaction mechanisms http://chemistry.berkeley.edu/links/reactions.html o Notice that there is a point where the middle molecule is not bonded to either. This is higher energy and therefore qualifies as the “activated complex” http://neon.cm.utexas.edu/academic/resources_movies/movies/iverson/main.ht m o Notice here that there is a point where all three molecules are bonded together. This is high energy because all the electrons are getting too close! Therefore, this qualifies as an activated complex as well. Activation Energy Energy H < 0 Reaction Coordinate The diagram above shows that The change in energy between reactants and products is the change in enthalpy The reaction is exothermic The reaction requires some energy before it will start, Activation Energy: Ea Example of Activation Energy: 1. Candles don’t burn until you ignite them 2. Matches don’t light until you use some of your own energy as you strike them 3. The food didn’t burn until it was lit 4. The Nitrogen triiodide (remember explosion video) doesn’t explode until it was tickled Analogy: Old Love, New Love Reactants are like you and an old boyfriend/girlfriend…content until you break up. You are in a state of agitation…activated complex…until you meet and bond with your new boy/girl and you are happy…at a low energy state….a new product has been formed. What would an endothermic reaction look like? Activation Energy Energy H > 0 Reactants Reaction Coordinate ENDOTHERMIC REACTION What is the likelihood of an endothermic reaction taking place? Not, likely Therefore we say it’s non-spontaneous Spontaneous: refers to the tendency of a reaction to occur o Spontaneous does not refer to how fast o Carbon Diamond is spontaneous but takes forever! o Rusting spontaneous but takes a long time! We already know that substances like to be in a lower energy state… Therefore, exothermic reactions are usually spontaneous Why would a reaction that creates substances at a higher energy ever happen? Stay tuned… 1. 2. 3. 4. 5. 6. Which are exothermic? Which are endothermic? Which require activation energy? Which are spontaneous? Which reaction could provide the most energy? Which reaction would require the most energy? A D B C E