Reaction Energy & Rate Worksheet
advertisement
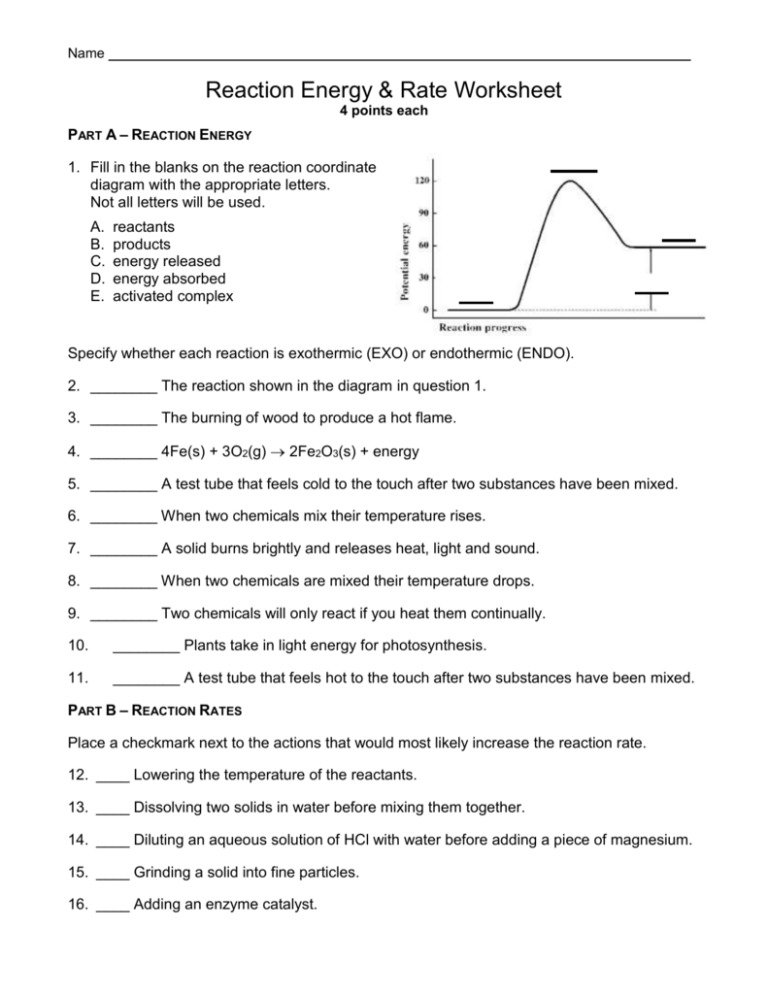
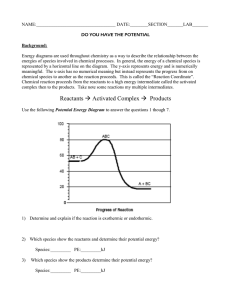
Name _____________________________________ Reaction Energy & Rate Worksheet 4 points each PART A – REACTION ENERGY 1. Fill in the blanks on the reaction coordinate diagram with the appropriate letters. Not all letters will be used. A. B. C. D. E. reactants products energy released energy absorbed activated complex Specify whether each reaction is exothermic (EXO) or endothermic (ENDO). 2. ________ The reaction shown in the diagram in question 1. 3. ________ The burning of wood to produce a hot flame. 4. ________ 4Fe(s) + 3O2(g) 2Fe2O3(s) + energy 5. ________ A test tube that feels cold to the touch after two substances have been mixed. 6. ________ When two chemicals mix their temperature rises. 7. ________ A solid burns brightly and releases heat, light and sound. 8. ________ When two chemicals are mixed their temperature drops. 9. ________ Two chemicals will only react if you heat them continually. 10. ________ Plants take in light energy for photosynthesis. 11. ________ A test tube that feels hot to the touch after two substances have been mixed. PART B – REACTION RATES Place a checkmark next to the actions that would most likely increase the reaction rate. 12. ____ Lowering the temperature of the reactants. 13. ____ Dissolving two solids in water before mixing them together. 14. ____ Diluting an aqueous solution of HCl with water before adding a piece of magnesium. 15. ____ Grinding a solid into fine particles. 16. ____ Adding an enzyme catalyst. PART C – REACTION COORDINATE DIAGRAM 17. The reaction shown above is exothermic / endothermic. 18. Which letter represents the energy of the reactants? _______ 19. Which letter represents the energy of the products? _______ 20. Which letter represents ΔH for the catalyzed reaction? _______ 21. Which letter represents ΔH for the uncatalyzed reaction? _______ 22. Which letter represents the activation energy for the catalyzed reaction? 23. Which letter represents the activation energy for the uncatalyzed reaction? _______ 24. Which letter represents the energy of the activated complex for the catalyzed reaction? _______ 25. _______ Which letter represents the energy of the activated complex for the uncatalyzed reaction? _______