Molecular Structures
advertisement

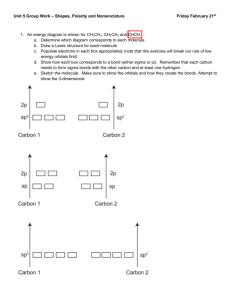
Molecular Structures PURPOSE: To construct and to examine models of hybrid orbitals and to investigate the geometry associated with them. APPARATUS AND CHEMICALS: Models THEORY AND PROCEDURE: Your laboratory instructor will show you the models they represent atoms, s, p, d, and hybrid orbitals that will be used. A.sp orbitals s and p atomic orbitals hybridize to give two sp hybrid orbitals. The geometry of sp hybrids formed is linear. Experimentally it is determined that BeCl2 is a linear molecule with two identical Be-Cl bonds. 1. Show by writing electron box diagrams for the Be atom in its ground and excited states, and how it can hybridize to form sp hybrids. (Atomic number of Be is 4) 2. Construct BeCl2 molecule. B. sp2 orbitals One s and two p atomic orbitals hybridize to give three sp2 hybrid orbitals. The geometry of sp2 hybrids formed is triangular planar. B.I. Experimentally it is determined that BF3 is a triangular planar molecule with both F-B-F angles equal to 1200. 1. Show by writing electron box diagrams for the B atom in its ground and excited states, and how it can hybridize to form, sp2 hybrids. (Atomic number of B is 5) 2. Construct a BF3 molecule. B.II. If a lone pair occupies one of the sp2 orbitals the the goemtry is angular or V-shaped. The lone pair elevtrons tend to repel the bond pair electrons more than the bond pair electrons repel each other. Thus the angle between bond pair electrons decreases. Experimentally it is determined that in the gas phase SnCl2 is an angular molecule with Cl-Sn-Cl angle slightly less than 1200, and both bonds in length and strength. 1. Show by writing electron box diagrams for the valence elctrons of Sn atom in its ground and excited states, and how it can hybridize to form sp2 hybrids. (Sn in group IVB in the 5th period ) 2. Remove one electron of the F atoms in BF3 molecule to construct a SnCl2 molecule. C. sp3 orbitals One s and three p atomic orbitals hy6bridize to give four sp3 hybrid orbitals. The geometry of sp3 hybrid formed is tetrahedral. C.I. Experimentally it is determined that CH4 is a tetrahedral molecule with all H-C-H angles equal to 109.50. 1. Show by writing eletron box diagrams for the C atom in its ground and excited states, and how it can hybridize to form sp3 hybrids. (Atomic number of C is 6) 2. Construct a CH4 molecule. C.II. If a lone pair occupies one of the sp3 orbitals then the geometry is trigonal pyramidal. The lone pair electrons (LP-BP) tend to repel the bond pair electrons more than the bond pair electrons (BP-BP) repel each other. Thus the angle between bond pair electrons decreases. Experimentally it is determined that NH3 has a trigonal pyramidal structure and H-N-H angles are 107.30. all N-H bonds are equal in length and strength. 1. Show by writing electron box diagrams for the electrons of N atom in its ground and excited states, and how it can hybridize to form sp3 hybrids.(N is in group VA.) 2. Remove one of the H atoms in CH4 molecule to construct a NH3 molecule. C.III. If a second lone pair occupies another sp3 orbital then the geometry is angular or Vshaped. The repulsions between two lone pairs are greater than repulsion between lone pair (LP-LP) and a bond pair (BP-BP). Thus the angle between bond pair electronsdecreases further. Experimentally it is determined that H2O is an angular molecule and both H-O bonds are equivalent . The H-O-H angle is 104.50. 1. Show by writing electron box diagrams for the electrons of O atom in its ground and excited states, and how it can hybridize to form sp3 hybrids.(O is in group VIA.) 2. Remove one of the H atoms in NH3 molecule to construct a H2O molecule. D.sp3d orbitals One s, three p and one d atomic orbitals hybridize to give five sp3d hybrid orbitals. The geometry of sp3d hybrids formed is trigonal bipyramidal. D.I. Experimentally it is determined that in PCl5 molecule the three P-Cl bonds lie in a plane at 1200 to each other while the ather two bonds are slightly longer and perpendecular to the three bonds in the plane. 1. Show by writing electron box diagrams for the electrons of P atom in its ground and excited states, and how it can hybridize to form sp3d hybrids.(O is in group VIA.) 2. Construct a PCl5 molecule. D.II. If a lone pair occupies one of the sp3d orbitals then the geometry is distorted tetrahedron (irregular tetrahedron). Explain considering LP-BP repulsion at 900. Experimentally determined geometry shows TeCl4 molecule to be a distorted tetrahedron. 1. Show by writing electron box diagrams for the valence electrons of Te atom in its ground and excited states, and how it can hybridize to form sp3d hybrids. 2. Remove one of the Cl atoms in PCl5 molecule to construct a TeCl4 molecule.(Te is a group VIB element.) D.III. If a second lone pair occupies another sp3d orbitals then the geometry is T-shaped. Explain considering LP-LP and LP-BP repulsions. Experimentally it is determined that BrF3 is a T-shaped molecule with bond angles of 87.50. 1. Show by writing electron box diagrams for the valence electrons of Br (Br is group VIIA element) in its ground and excited states, and how it can hybridize to form sp3d hybrids. 2. Remove one of the Cl atoms in TeCl4 molecule to construct a BrF3 molecule. D.IV. If a third lone pair occupies another sp3d orbitals then the geometry is linear. Eplain considering LP-LP and LP-BP repulsions. Experimentally it is determined that XeF2 is a linear molecule. 1. Show by writing electron box diagrams for the valence electrons of Xe (Xe is group VIIIA element) in its ground and excited states, and how it can hybridize to form sp3d hybrids. 2. Remove one of the F atoms in BrF3 molecule to construct a XeF2 molecule. E. sp3d2 orbitals One s, three p, two d atomic orbitals hybridize to give five sp3d2 hybrid orbitals. The geometry of sp3d2 hybrids formed is octahedral. E.I. Experimentally it is determined that in SF6 molecule is octahedral. All bond angles are 900. 1. Show by writing electron box diagrams for the electrons of S atom in its ground and excited states, and how it can hybridize to form sp3d2 hybrids.(O is in group VIA.) 2. Construct a SF6 molecule. E.II. If a lone pair occupies one of the sp3d2 orbitals then the geometry is square pyramidal. Explain considering LP-BP repulsion at 900. Experimentally determined geometry shows IF5 molecule to be a sqyare pyramidal.(I is a group VIIA element.) 1. Show by writing electron box diagrams for the valence electrons of one I in its ground and excited states, and how it can hybridize to form sp3d2 hybrids. 2. Remove one of the F atoms in SF6 molecule to construct a IF5 molecule. E.III. . If a second lone pair occupies another sp3d2 orbitals then the geometry is square planar. Explain considering LP-LP and LP-BP repulsions. Experimentally it is determined that XeF4 is a sqyare planar molecule with bond angles of 900. 1. Show by writing electron box diagrams for the valence electrons of Xe (Br is group VIIA element) in its ground and excited states, and how it can hybridize to form sp3d hybrids. 2. Remove one of the Cl atoms in TeCl4 molecule to construct a BrF3 molecule. F. Simple organic molecules and conformations. 1. Construct a methane, CH4, molecule. 2. Construct a second sp3 orbital model. Demonstrate that an sp3 orbital on one carbon overlap with an sp3 orbital on the second linking the two C atoms. 3. Construct an ethane, CH3-CH3, molecule. 4. Viex the model from the end. Note that rotating one C atom about C-C bond results in different conformations. Draw the end point and corresponding side view projections. 5. Replace two of the H atoms by others to construct 1,1-dichloroethane and 1,2 – dichloroethane. How many principal conformations are possible for each structure? G. Complete the Table of Molecular Geometry. REVIEW QUESTIONS: Before beginning this experiment in the laboratory, you should be able to answer the following questions: 1. Distinguish between molecular and structural formulas. 2. What is a condensed structural formula? Give an example. 3. What is the meaning of word isomer? 4. Why should the properties of structural isomers differ? 5. Draw the structural formulas for ethane and propane. 6. Distinguish between molecular and emprical formula. 7. The molecular formula of benzene C6H6. What is the emprical formula of benzene? 8. Distinguish between geometric and structural isomerism. 9. Carbon has a valence of 4, oxygen 2, and hydrogen 1. How many compounds of C, H, and O containing only one carbon and one oxygen can you make? Draw their structures. 10. Draw Lewis electron dot formulas for the compoounds in question 9 and name them.