POSTLAB 7
advertisement

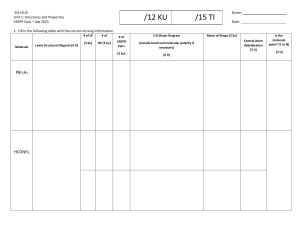
EXPERIMENT • VSEPR THEORY AND SHAPES OF MOLECULES Name__________________________________________ Lab Instructor______________________________ Date___________________________________________ Lab Section________________________________ Postlaboratory Exercise 1. Draw all of the resonance structures for N2O5 2. Which of these are the major resonance contributors? 3. C6H6 is a ring-shaped molecule. Draw a lewis dot structure for this molecule. Based on what you have drawn, do you think this molecule is planar, in that all of its atoms are on the same plane? Why or why not?