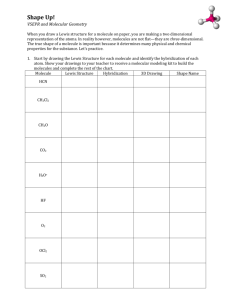
Unit5 - hybridization, molecule shape and properties
advertisement
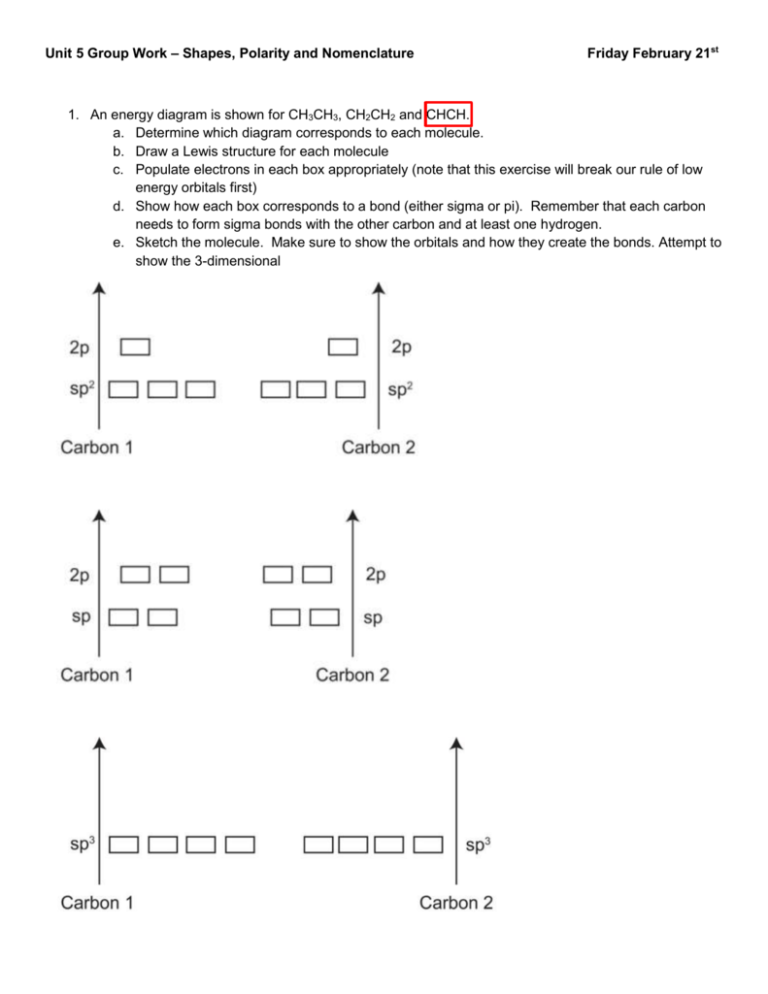
Unit 5 Group Work – Shapes, Polarity and Nomenclature Friday February 21st 1. An energy diagram is shown for CH3CH3, CH2CH2 and CHCH. a. Determine which diagram corresponds to each molecule. b. Draw a Lewis structure for each molecule c. Populate electrons in each box appropriately (note that this exercise will break our rule of low energy orbitals first) d. Show how each box corresponds to a bond (either sigma or pi). Remember that each carbon needs to form sigma bonds with the other carbon and at least one hydrogen. e. Sketch the molecule. Make sure to show the orbitals and how they create the bonds. Attempt to show the 3-dimensional 2. Why can’t Hydrogen form hybrid orbitals? 3. Name each of the following compounds. a. HCl b. CCl4 c. NaCl d. MgF2 e. HNO3 f. HNO2 g. H2CO3 h. CO32i. CO2 j. Cu2O k. CO l. FeCl2 4. For each of the boldfaced molecules in Problem 3, draw a correct Lewis structure and determine the shape and hybridization around each non-hydrogen atom. 5. For each of the following compounds: a. draw a Lewis Structure b. State the hybridization on all non-hydrogen atoms c. Determine the shape around each central atom. d. Identify all polar bonds. e. Indicate molecule polarity (if applicable) f. Identify all intermolecular forces that can stabilize the molecule in condensed phases. HCl H2O CH3OCH3 CF3Br HCN CCl4 6. Based on IM force strength, rank these molecules in order of increasing melting temperature. Recall that stronger IM forces melt at higher temperatures. F2 HF H2 BrF NaF CH3 CH2CH2CH3 7. Determine the hybridization at each non-hydrogen atom and indicate the shape around each central atom. Note that lone pairs are not necessarily shown.