Fl&o_5
advertisement
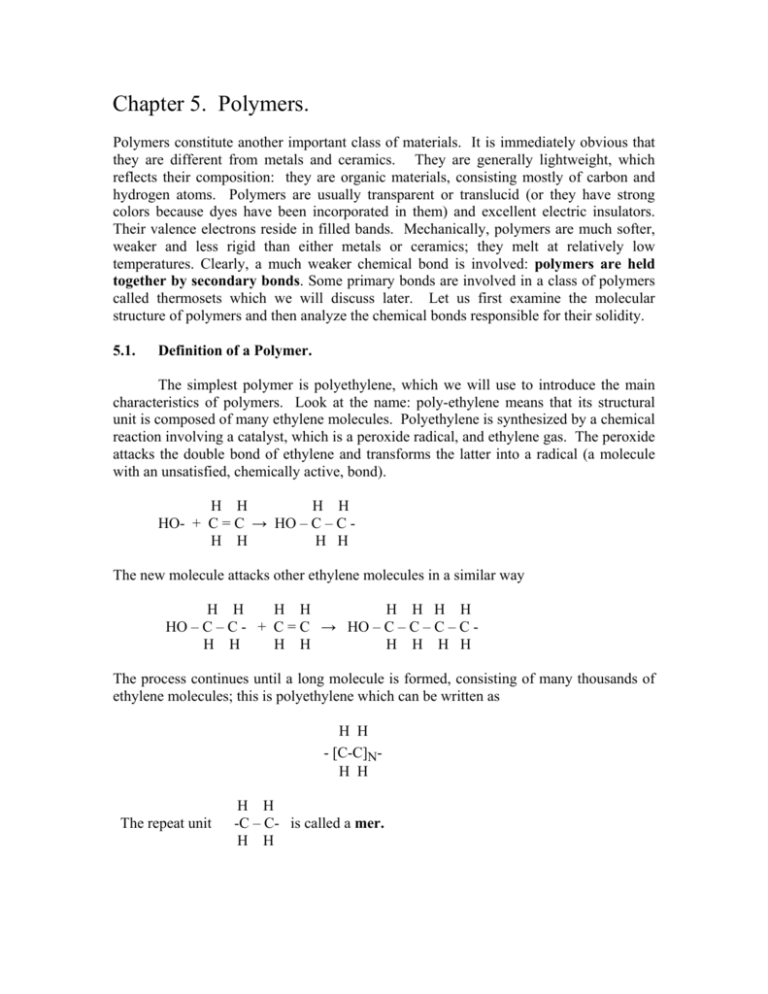
Chapter 5. Polymers. Polymers constitute another important class of materials. It is immediately obvious that they are different from metals and ceramics. They are generally lightweight, which reflects their composition: they are organic materials, consisting mostly of carbon and hydrogen atoms. Polymers are usually transparent or translucid (or they have strong colors because dyes have been incorporated in them) and excellent electric insulators. Their valence electrons reside in filled bands. Mechanically, polymers are much softer, weaker and less rigid than either metals or ceramics; they melt at relatively low temperatures. Clearly, a much weaker chemical bond is involved: polymers are held together by secondary bonds. Some primary bonds are involved in a class of polymers called thermosets which we will discuss later. Let us first examine the molecular structure of polymers and then analyze the chemical bonds responsible for their solidity. 5.1. Definition of a Polymer. The simplest polymer is polyethylene, which we will use to introduce the main characteristics of polymers. Look at the name: poly-ethylene means that its structural unit is composed of many ethylene molecules. Polyethylene is synthesized by a chemical reaction involving a catalyst, which is a peroxide radical, and ethylene gas. The peroxide attacks the double bond of ethylene and transforms the latter into a radical (a molecule with an unsatisfied, chemically active, bond). H H H H HO- + C = C → HO – C – C H H H H The new molecule attacks other ethylene molecules in a similar way H H H H H H H H HO – C – C - + C = C → HO – C – C – C – C H H H H H H H H The process continues until a long molecule is formed, consisting of many thousands of ethylene molecules; this is polyethylene which can be written as H H - [C-C]NH H The repeat unit H H -C – C- is called a mer. H H The number N of mers is the degree of polymerization, this can be very large, often reaching N = 10,000, resulting in molecules as larger than one micrometer. These are called macromolecules. The molecular weight of the polymer is the sum of all atomic weights, in other words it is N times the molecular weight of the mer. The molecular weight of ethylene is M = 2x12 + 4x1 = 28. With N = 10,000, the molecular weight of the polyethylene chain is 280,000. In the processing of polymers, it is not possible to obtain all chains of the same length. Thus, in practice, the molecular weight given for a polymer is an average value. 5.2. Polymers and secondary bonds; thermoplastics. Now let us look at the bonding of polyethylene. The bonds between the carbon atoms in the chain (red in fig. 5.1) and between the carbon and hydrogen are covalent and based on the sp3 hybrid valence electrons of carbon. This results in the shape of the molecule shown in figure 5.1. These valence electrons completely fill the valence band; therefore polymers are insulators and are often transparent. Figure 5.1. Portion of a polyethylene molecule. The white atoms are carbon; the dark atoms are hydrogen. Covalent bonds are sketched in red and blue. When two polyethylene molecules approach, there is no sharing of valence electrons between them. The bonding between the chains is a weak van der Waals bond. (In the van der Waals bond, the charges of the valence electrons in one of the molecules repel the electrons of the other so that the valence orbitals are slightly deformed. Small electric dipoles are induced in the two approaching molecules. These dipoles attract each other weakly). The van der Waals bond has a strength of about 10 kJ/mol or 0.1 eV/atom (see Table 1.2.); this is about 50 times weaker than the bond of metals and ceramics. Van der Waals bonds, for instance, occur between noble atoms which liquefy only at very low temperatures. The weakness of the van der Waals bond is responsible for the softness of polyethylene. Other polymers, such as polyvinyl chloride (PVC), polytetrafluoroethylene (PTFE which is commonly known by its trade name Teflon©) and polypropylene are built on the same principle but with different mers. These are shown in figure 5.2. Figure 5.2. Top: polyethylene, a) PTFE, b) PVC, c) polypropylene. Figure 5.3 shows portions of two polyvinyl chloride (PVC) molecules. The structure of PVC is similar to that of polyethylene, except that one of the four hydrogen atoms is replaced by chlorine. Chlorine has a high electronegativity and attracts electron charge, creating a mixed covalent – ionic bond. The electron charge that chlorine attracts to itself is removed from the rest of the mer, which is then positively charged. The result is the formation of a permanent electric dipole in each mer (marked in red). When two PVC molecules are in proximity, no electron transfer or exchange occurs between them. The electric dipoles of the molecules attract each other by virtue of electrostatic forces. This forms a secondary bond by permanent dipoles, which is about 5 times stronger than the van der Waals, but still 10 times weaker than the primary bonds described for metals and ceramics. The strength of the permanent dipole bond is responsible for the fact that PVC is much stronger than polyethylene. (Think of vinyl floors in kitchens). When one of the charged atoms in a permanent dipole bond is hydrogen, one speaks of a hydrogen bond. Such bonds, for instance, are responsible for the cohesion of water and ice. Figure 5.3. Portions of two polyvinyl chloride molecules in close proximity. The small dark circles are hydrogen, the white circles represent carbon atoms and the yellow circles marked – chlorine ions. Electric dipoles are formed in the mers because of the polarity of the C=Cl bond. This creates a physical, or secondary, bond between adjacent chains due to permanent dipoles. Van der Waals, permanent dipole and hydrogen bonds are secondary bonds. They are 10 to 50 times weaker than the primary bonds of metals and ceramics and account for the mechanical properties and the low melting temperatures (120 to 300 oC) of polymers. The polymers we have described are thermoplastics: when they are heated, their thermal energy overcomes the weak bonds between chains; the material becomes progressively softer until it liquefies. The term "thermoplastic" indicates that the materials can be deformed by heating. The real shape of a polyethylene molecule is not a straight bar as suggested by figure 5.1. While the sp3 hybrid rigidly imposes the angle of 109.5o between bonds, there is no real obstacle to rotation of a bond around its axis as illustrated in figure 5.4. Consequently, the polymer chains have an irregular shape sketched in figure 5.5. Figure 5.3. a) Possible rotation of the bond around its axis. As a consequence, the molecule can have the straight shape (b) or the irregular shape where the angle between bonds remains at 109.5o. Figure 5.4. Shape of a polymer molecule. (From L.R.G. Trelor, The Physics of Rubber Elasticity, 2nd edition, Oxford University Press, oxford, 1958, p.47) In a solid polymer, the individual chains are intertwined (like a plate of spaghetti). This intertwining of the molecules has a large influence on the mechanical properties of polymers. In particular, such polymers can not assume the structure of a crystal. They are amorphous. Consequently, they do not have a precise melting point. As in the case of glass, the polymer is liquid at high temperatures; its viscosity increases as the material cools and assumes large enough values that the material acts as a solid. 5.3. Thermosets. In another class of polymers, called thermosets, primary bonds are formed between the molecules. These primary bonds between chains are called crosslinks. Thermoset polymers are synthesized by a chemical reaction between two different substances. Usually, one of the substances consists of large hydrocarbon molecules and is called the resin. The other substance binds the resin molecules chemically together and is called the hardener. With primary (strong) bonds between the chains, thermosets are stronger and stable to somewhat higher temperatures than the thermoplastics. They do not soften at high temperatures but loose their hydrogen and transform into char at high temperature. As a consequence, thermosets are not recyclable. There is a push from the government to replace thermosets with thermoplastics as much as possible in order to permit recycling. Thermosets are used in some applications where high-temperature stability is essential. They are also used as matrix in fiber-reinforced composites (fiber-glass or graphite fibers) because the fabrication of large objects, such as boats, car bodies or airplane parts vastly benefits from the ability of a thermoset to solidify at room temperature. We now examine two important such thermosets. 5.3.1. Epoxy Epoxy glue sold in hardware stores comes in two tubes. When one mixes the content of the tubes, the glue hardens in a short time thanks to a chemical reaction between the two substances. The content of the tubes is an epoxy resin and an ethylene diamine hardener. The relevant chemical structure of epoxy is the epoxide group at its extremity This structure forms a double bond between two carbons that can be opened to bond with the hardener. A typical epoxy molecule has the form Note the degree of polymerization n. When the epoxy is mixed with ethylene diamine, the oxygen bond is opened and the reaction binds two epoxy molecules by covalent bonds. 5.3.2. Unsaturated Polyester. This thermoset, which is less expensive than epoxy, is widely used in fiberglass composites. The resin is a linear polyester and the hardener is styrene. The reaction between the two forms a network in which the molecules are joined with primary bonds. Note that C = C double bonds are opened in order to create valences available for the bonding of the molecules. In the figure, the open bonds shown by green arrows react with styrene to continue the network. 5.3.2. Rubber. Rubber is a natural or synthetic polymer with a molecular structure that allows it to stretch by large amounts. Natural rubber, which is cis-1,4 Polyisoprene has the following structure An isomer of this molecule, trans-1,4, polyisoprene has the structure This gutta-percha and is not a rubber. We note that the C =C double bond makes this mer flat. The actual shapes of these two molecules are and arc for rubber and straight molecule for gutta percha ` Rubber Gutta-percha The arched shape of the rubber mer permit the formation of a coiled polymer molecule that deforms in the same way as a coil spring. This is not possible with the straight guttapercha. The synthetic rubbers polybutadiene and polychloroprene (also called neoprene) have the same mer geometry and are elastic for the same reason. Vulcanization. Rubber is a thermoplastic polymer with secondary bonds between the chains. Natural rubber is too soft for many applications. In 1844 Charles Goodyear obtained a patent for strengthening rubber by reacting it with sulfur. The process is called vulcanization of rubber. Sulfur reacts with the double bond in the rubber and establishes primary bonds between the chains, effectively transforming the rubber into a thermoset, as shown in figure 5.5. Vulcanization can be performed to any desired degree, producing rubber that is hard enough for automotive tires or hockey pucks. Figure 5.5. Vulcanization of rubber. (a); fragments of two chains of rubber. (b) Crosslinking of the chains by sulfur. (c) sketch of rubber chains with sulfur crosslinks. The crosslinking inhibits the elastic deformation of the chains and hardens the rubber.









