hw7_2010_11ans - Rose
advertisement

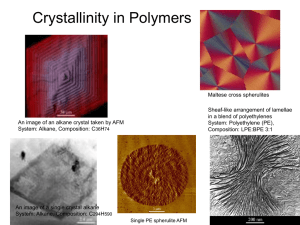
ME328 Materials Engineering Homework 7 At the following link, we find some good material on polymers from the Open University in the UK http://openlearn.open.ac.uk/course/view.php?id=2937 In figure 3 of Secion 1.1 Polymer types we see that polymers are divided into Thermoplastics and Thermosets. Thermoplastics can be “remelted” and reused while thermosets (like eggs) can be cooked only once. Both types of polymer have a combination of primary (e.g. covalent) and secondary (e.g. hydrogen) bonding in their microstructures. 1. What is the difference in microstructure between thermoplastics and thermosets? Thermo plastics have chains with primary bonds (e.g. covalent, ionic) along the chains and secondary bonds (e.g. hydrogen, vander waals) between the chains. Thermosets have primary bonds along the chains and both primary and secondary between the chains. The cross linkining (primary bonds) between the chains are extensive. In some thermosets the bonding is essentially more of a 3-D network rather than a chain structure. 2. How does the type of microstructural bonding lead to the irreversible curing characteristic of thermosets? The primary bonds between the chains are resistant to heating (up to the point of chain breakdown (burning, degradation). In thermoplastics, the secondary bonds are not so strong when the average molecular distance increases (heating) and so are able to flow in a liquid manner at higher temperatures. 3. What is the nature of the bonding in an elastomer and how does this contribute to the typical properties of elastomers (large scale elastic deformation)? In elastomers, there are relatively few primary bond cross links between chains that tend to be highly coiled. Significant uncoiling can occur with the bonds sliding past one another. This allows for large scale deformation. The elastic recovery is due to the ”entropy spring” we will discuss next week. Some polymers are fully amorphous and some polymers are partly crystalline. 4. Name 3 polymers that are fully amorphous. PS, PMMA,PC 5. Sketch and label (e.g. Glass transition temperature, Melting temperature) the Volume – Temperature graph for a fully amorphous polymer. Tg 6. Name 3 polymers that can be partly crystalline. PE, PP, PA, POM 7. Sketch and label (e.g. Glass transition temperature, Melting temperature) the Volume – Temperature graph for a partly crystalline polymer. Tg Tm 8. A number of factors affect the ability of a polymer to be crystalline. How do each of the following affect the degree of crystallinity? a) Increased chain branching (increases, decreases, doesn’t change) degree of crystallinity. b) A atactic structure (increases, decreases, doesn’t change) degree of crystallinity. c) A random copolymer structure (increases, decreases, doesn’t change) degree of crystallinity. d) Increased cross-linking (increases, decreases, doesn’t change) degree of crystallinity. 9. For a greater degree of crystallinity a) yield strength will (increase, decrease, remain the same) b) shrinkage when molding will (increase, decrease, remain the same) c) Young’s modulus will (increase, decrease, remain the same) 10. Describe the effect of chain length, polar side groups, bulky side groups, and backbone atoms on strength and stiffness. Strength and stiffness in thermoplastic polymers tends to result when chain motion becomes more difficult. Chain Length – Increased length leads to increased entanglement and more difficult relative motion between chains. Polar Side Groups – Stronger secondary bonds between chains makes relative chain motion more difficult. Bulky side groups – Bulky side groups create entanglement and reduce chain motion in the same manner that entangled barbed wire is harder to pull apart than smooth wire. Changing backbone atoms or backbone structures can make bond rotations more difficult than the carbon-carbon bond. This can also inhibit chain uncoiling.