Honors Atomic Mass Practice
advertisement

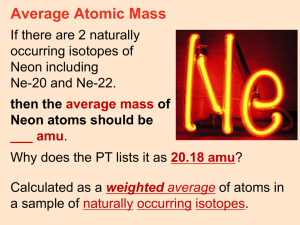
Honors Atomic Mass Practice Name: ____________________________________________ Date: _______________ 1. Naturally occurring bromine is a mixture of bromine-79 and bromine-81. Estimate the isotopic composition of naturally occurring bromine. 2. Calculate the atomic mass of naturally occurring neon, which is a mixture of 90.48% neon-20 (atomic mass = 19.99 amu), 0.27% neon-21 (atomic mass = 20.99 amu), and 9.25% neon-22 (atomic mass = 21.99 amu.) 3. The path of which of the following ions is bent the most in a mass spectrometer: 12 H 1 or 11H 1 ? Explain your answer. 4. It can be shown using a mass spectrometer that the ratio of naturally occurring chlorine-35 to its isotope chlorine-37 is 3:1. Assuming that no other isotope exists, what is the atomic weight of chlorine? 5. A sample of element X has an atomic mass of 67.09 amu. It contains three isotopes. The first has a mass of 68.0 amu and is found with 3.0% abundance. The second has a mass of 70.0 amu and a 2.0% abundance. If the third has a mass of 67.0 amu, what is its percent abundance? 6. A sample of element X contains 70% of 25X atoms, 20% of 27X atoms, and 10% of the third isotope. The reported atomic mass of the element is 25.70 amu. What is the mass of the third isotope? 7. 35Cl and 37Cl are the only naturally occurring chorine isotopes. What percentage distribution accounts for the atomic weight of 35.453? 8. To account for nitrogen’s atomic weight of 14.0067, what must the ratio of 15N to 14N atoms be in natural nitrogen?