weighted average atomic mass
advertisement

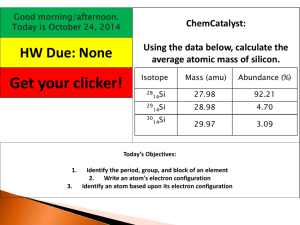
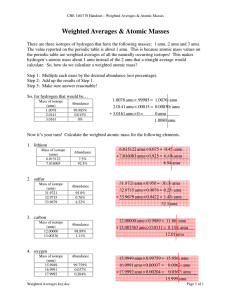
Average Atomic Mass If there are 2 naturally occurring isotopes of Neon including Ne-20 and Ne-22. then the average mass of Neon atoms should be ___ amu. Why does the PT lists it as 20.18 amu? Calculated as a weighted average of atoms in a sample of naturally occurring isotopes. Average Atomic Mass A weighted average atomic mass is calculated from both the masses of each isotope and the relative abundance (%) of each isotope. 63Cu is 69% abundant 65Cu is 31% abundant To which will the average mass be closer, 63 or 65? copper-63 has a mass of 62.93 amu and natural abundance of 69.15% copper-65 has a mass of 64.93 amu and has a natural abundance of 30.85% Calculating Average Atomic Mass Avg. Mass = (Mass1)(%) + (Mass2)(%) … = (62.93)(0.6915) + (64.93)(0.3085) = 63.55 neon-20 neon-22 Which isotope is in greater relative abundance in natural samples of neon? because… the weighted average atomic mass of neon from the PT is 20.18 amu. Quick Quiz! 1. If an unusually selected sample of sulfur contained 90.0% sulfur-32 and 10.0% sulfur-34, what would be its average atomic mass? A. 32.2 amu B. 32.9 amu C. 33.5 amu D. 34.2 amu Avg. = (M1)(%) + (M2)(%) = (32)(0.900) + (34)(0.100) = 32.2