Naming Chemical Compounds Worksheet
advertisement

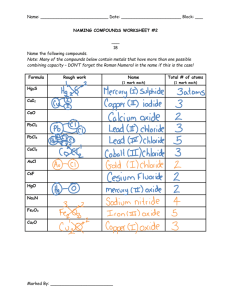
Name _______________________ Date _____________ Per ______ Mixed Practice: Names and Formulas-ANSWERS First determine whether each of the following compounds is ionic (M + NM), polyatomic (3 or more atoms), or covalent (2 NM’s). Then write the name for each compound. 1) NO2 mononitrogen dioxide 2) NaBr sodium bromide 3) SiO2 monosilicon dioxide 4) P2Br4 diphosphorous tetrabromide 5) Fe2O3 iron oxide (could have roman numerals here, but I’m okay if you don’t) 6) SF6 monosulfur hexafluoride 7) Li2S lithium sulfide 8) MgBr2 magnesium bromide 9) N2S dinitrogen monosulfide 10) BeBr2 berryllium bromide 11) SO3 monosulfur trioxide 12) Cu2S copper sulfide 13) BF3 monoboron trifluoride 14) I2 skip First determine whether each of the following compounds is ionic (M + NM), polyatomic (3 or more atoms), or covalent (2 NM’s). Then write the formula for each compound. 15) carbon monosulfide CS 16) vanadium(II) phosphide SKIP 17) oxygen difluoride OF2 18) gold(I) iodide AuI 19) triboron tetrahydride B3H4 20) aluminum fluoride AlF3 21) dinitrogen heptoxide N2O7 22) dinitrogen trioxide N2O3 23) cadmium chloride SKIP 24) aluminum oxide Al2O3 25) disulfur trichloride N2S3 26) cobalt(II) oxide CoO 27) pentaphosphorus hexafluoride P5F6 Naming Chemical Compounds Name the following ionic compounds: 1) 2) 3) 4) NaBr CaO Li2S MgBr2 Write the formulas for the following ionic compounds: 6) 7) 8) potassium iodide magnesium oxide aluminum chloride 12) beryllium phosphide 16) calcium chloride 18) aluminum oxide Write the names of the following covalent compounds: 21) 22) 23) SO3 N2S PH3 24) 25) 26) BF3 P2Br4 CO 27) 28) 30) SiO2 SF6 NO2 Write the formulas of the following covalent compounds: 31) 32) 33) 34) 36) 37) 38) 39) 40) nitrogen trichloride boron monocarbide dinitrogen trioxide phosphorus pentafluoride sulfur dibromide diboron tetrahydride oxygen difluoride carbon disulfide nitrogen monoxide