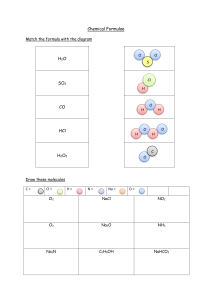
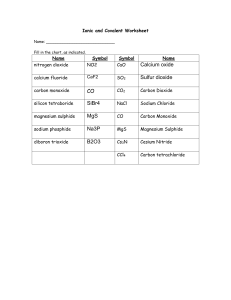
Naming Compounds Worksheet #2
advertisement

Name: __________________________ Date: _______________________ Block: ___ NAMING COMPOUNDS WORKSHEET #2 ___ 18 Name the following compounds. Note: Many of the compounds below contain metals that have more than one possible combining capacity – DON’T forget the Roman Numeral in the name if this is the case! Formula Rough work Hg2S CuI2 CaO PbCl2 PbCl4 CoCl2 AuCl CsF HgO Na3N Fe2O3 Cu2O Marked By: ________________________ Name Total # of atoms (1 mark each) (1 mark each) Name: __________________________ Date: _______________________ Block: ___ WRITING CHEMICAL FORMULAE WORKSHEET #2 ___ 30 Write the chemical formulae for the following compounds. Show all your work! Chemical Name Rough work Tin (II) oxide Lead (IV) fluoride Iron (III) sulphide Calcium bromide Lead (IV) bromide Lithium chloride Mercury (II) chloride Yttrium nitride Zirconium oxide Lead (IV) sulphide Titanium (III) oxide Vanadium (V) phosphide Chromium (VI) oxide Manganese (II) oxide Magnesium oxide Copper (II) nitride Palladium (IV) oxide Palladium (II) oxide Copper (I) nitride Silver iodide Marked By: ________________________ Formula Total # of atoms (1 mark each) (½ mark each)