Empirical formula
advertisement

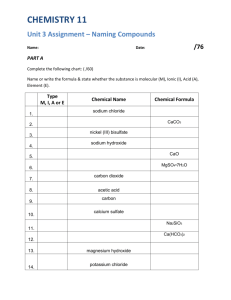
NOMENCLATURE Chapter 2 Chemical Formulas • Formula Unit: • Identifies exact number of atoms in an ionic compound • Molecular formula: • Identifies exact number of atoms in a covalent molecule • Empirical formula: • Shows the simplest whole number ratio of atoms in a compound molecular H2O C6H12O6 empirical H2O CH2O O3 O N2H4 NH2 DIATOMIC ELEMENTS Some elements do not like to be alone… so they bond to themselves! HOFBrINCl Ionic Compounds • consist of a combination of cations and anions formed from a transfer of electrons • the formula unit is always identical to the empirical formula • the sum of the charges on the cation(s) and anion(s) in each formula unit must equal zero The ionic compound NaCl NAMING BINARY IONIC COMPOUNDS RULE 1 Metal – Nonmetal 1. Write the metal (positive ion) first 2. Change the ending of the second word to -ide Rule 1 Examples 1. KBr 2. CaBr2 3. LiF 4. Li2O 5. MgO 6. BaS 7. K3P 8. Na3N 1. 2. 3. 4. 5. 6. 7. 8. Potassium Bromide Calcium Bromide Lithium Fluoride Lithium Oxide Magnesium Oxide Barium Sulfide Potassium Phosphide Sodium Nitride NAMING COMPOUNDS WITH POLYATOMICS RULE 2 Polyatomic Ions 1. DO NOT CHANGE ANYTHING! 2. When you have NH4 and a single element, change the second word to -ide Rule 2 Examples 1. 2. Ba(SO3) 3. Na2(CO3) Na(HCO3) 4. (NH4)3(PO4) 5. (NH4)(OH) 6. 1. Ba(SO4) 2. 3. 4. 5. 6. Barium Sulfate Barium Sulfite Sodium Carbonate Sodium Bicarbonate Ammonium Phosphate Ammonium Hydroxide Formula of Ionic Compounds 2 x +3 = +6 Al3+ Al2O3 1 x +2 = +2 Ca2+ 3 x -2 = -6 O2- 2 x -1 = -2 Ca Br2 1 x +2 = +2 1 x -2 = -2 Na2CO3 Na+ Br- CO32- NAMING COMPOUNDS WITH TRANSITION METALS RULE 3 Transition Metals 1. Can have more than one type of charge 2. Write the charge number in roman numerals Rule 3 Examples 1. Cu2O 2. CuO 3. FeCl2 4. FeCl3 5. SnCl4 6. Mn2O3 7. PbS 1. 2. 3. 4. 5. 6. 7. Copper (I) Oxide Copper (II) Oxide Iron (II) Chloride Iron (III) Chloride Tin (IV) Chloride Manganese (III) Oxide Lead (II) Sulfide NAMING HYDRATES • What is a hydrate? • Hydrates are named: Compound • Prefix-Hydrate FeCl3 • 6H2O = Iron (III) Chloride Hexahydrate Name to Formula – Criss Cross Rule 1. Lithium Fluoride 2. Sodium Sulfide 3. Aluminum Bromide 4. Iron (III) Oxide 5. Carbonic Acid 6. Calcium Carbonate 7. Magnesium Acetate 8. Copper (II) Sulfate Pentahydrate 9. Ammonium Sulfite 10. Barium Hypochlorite Covalent Compounds • Referred to as molecules • Consists of nonmetals covalently bonded • nonmetals or nonmetals + metalloids • Least electronegative element is usually written first NAMING COVALENT COMPOUNDS RULE 4 Nonmetal – Nonmetal USE PREFIXES! 1. Change the ending of the second word to -ide 2. No mono on the first word 3. Drop any double vowels NO2 nitrogen dioxide N2O dinitrogen monoxide TOXIC! Laughing Gas Prefixes Number of Atoms 1 2 3 4 5 6 7 8 9 10 Prefixes Rule 4 Examples 1. CO 2. CO2 3. SO2 4. SO3 5. N2H4 6. N2O3 7. PCl3 8. SiO2 9. P2O5 10. CS2 11. Al2O3 1. Carbon Monoxide 2. Carbon Dioxide 3. Sulfur Dioxide 4. Sulfur Trioxide 5. Dinitrogen Tetrahydride 6. Dinitrogen Trioxide 7. Phosphorus Trichloride 8. Silicon Dioxide 9. Diphosphorus Pentoxide 10. Carbon Disulfide 11. Aluminum Oxide ACIDS • a substance that yields hydrogen ions (H+) when dissolved in water. • example: hydrochloric acid refers to a water solution of the molecular compound hydrogen chloride, HCl • Many polyatomic ions are produced by the loss of hydrogen ions from oxyacids. Acid Name: sulfuric acid nitric acid phosphoric acid Polyatomic Ion: H2SO4 HNO3 H3PO4 sulfate nitrate SO24 phosphate PO34 NO3 NAMING ACIDS • Binary acids: consist of two elements, usually hydrogen and a halogen • “HX” • Oxyacids: contain hydrogen and a polyatomic ion (usually oxygen and a third nonmetallic element) • “HXO” NAMING BASES • Base: a substance that yields hydroxide ions (OH-) when dissolved in water. NaOH sodium hydroxide KOH potassium hydroxide Ba(OH)2 barium hydroxide