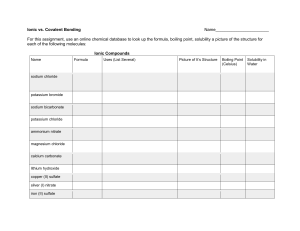
Ionic vs. Covalent Compounds Lab Worksheet
advertisement
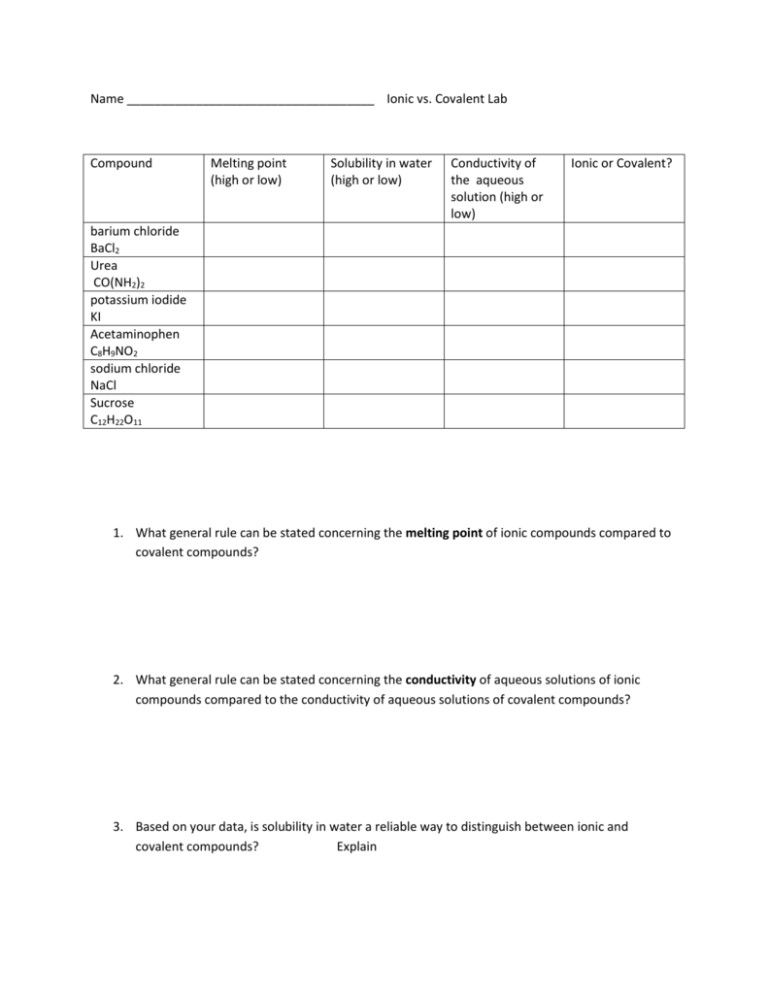
Name ____________________________________ Ionic vs. Covalent Lab Compound Melting point (high or low) Solubility in water (high or low) Conductivity of the aqueous solution (high or low) Ionic or Covalent? barium chloride BaCl2 Urea CO(NH2)2 potassium iodide KI Acetaminophen C8H9NO2 sodium chloride NaCl Sucrose C12H22O11 1. What general rule can be stated concerning the melting point of ionic compounds compared to covalent compounds? 2. What general rule can be stated concerning the conductivity of aqueous solutions of ionic compounds compared to the conductivity of aqueous solutions of covalent compounds? 3. Based on your data, is solubility in water a reliable way to distinguish between ionic and covalent compounds? Explain