ionic bonding practice worksheet - Holding
advertisement

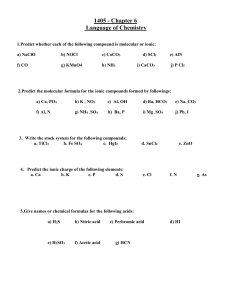
Name: _________________________________________ Date: ___________________ Ionic Bonding Practice Worksheet Regents Chemistry Directions: With your desk partner, draw the Lewis dot structures for each ion, indicating which ion loses an electron and which gains an electron. 1. CaO 2. K2S 3. Na2SO4 4. AlPO4 5. Li2O 6. BaCl2 7. KNO3 8. MgSO4 9. Mg3N2 10. AlBr3 11. What are the characteristics of ionic compounds? 12. Explain why ionic compounds can conduct electricity when melted and when in aqueous solutions. 13. Which pairs of elements are likely to form ionic compounds? a. Chlorine and bromine b. Potassium and helium c. Lithium and chlorine d. Iodine and sodium