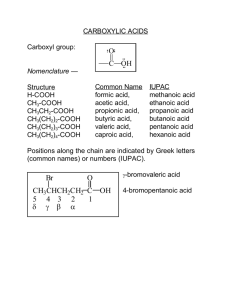
Carboxylic Acids
advertisement

SCH4C – Organic Chemistry 533580079 Date: _______________________ Carboxylic Acids Uses Acetic acid Citric acid Oxalic acid (rust remover and brass cleaner) ASA (Aspirin) Vitamin C (ascorbic acid) Has a ___________________________ (-COOH, typically shown as C=O and –OH on the same C atom) General Form Naming Carboxylic Acids Take parent name of alkane chain, drop the ‘e’, and replace it with ‘_____________’ The carboxylic acid group is _________________, so there is no need to note the position When numbering the carbon chain, the carbon in the carboxyl group is always numbered ____________________ Page 1 of 3 SCH4C – Organic Chemistry 533580079 Drawing Carboxylic Acids butanoic acid hexanoic acid 2-methylpentanoic acid 3-chloropropanoic acid Properties of Carboxylic Acids As with all other acids, they turn _________________________________________ The presence of the –OH group makes them polar Due to polarity, have _______________________________ Page 2 of 3 SCH4C – Organic Chemistry 533580079 Draw structures for the following compounds. 1. 3,3-diethyloctanoic acid 2. 2-iodo-4-methylhexanoic acid 3. 2,2-dimethylpentanoic acid 4. 2-ethylhexanoic acid 5. 3-chlorobutanoic acid 6. heptanoic acid 7. 2-pentenoic acid 8. ethanoic acid (commonly known as acetic acid or vinegar) 9. 2-chlorobutanoic acid 10. 2-bromo-3,3-dimethylbutanoic acid Name the following structures. 1. 5. CH2=CH-CH=CH-COOH ICH2COOH 2. 6. (CH3)3-C-COOH CH3(CH2)8COOH 3. 7. CH3 | CH3-CH2-CH-CH-COOH | CH3 (CH3)2CHCHCH2COOH | CH3 4. 8. BrCH2CH2CH2CH2CH2COOH CH2CHCH2COOH Page 3 of 3