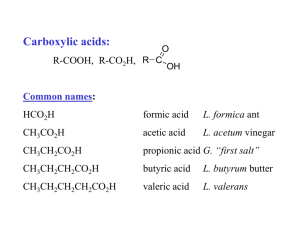
Carboxylic Acids
advertisement
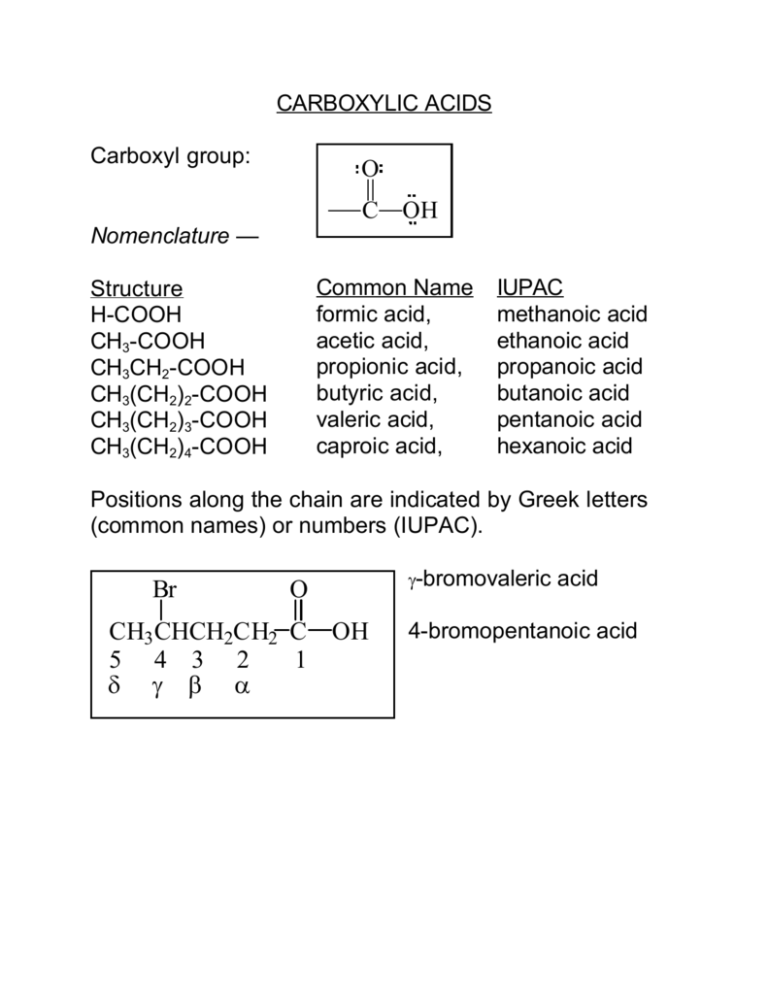
CARBOXYLIC ACIDS Carboxyl group: O C OH Nomenclature — Common Name formic acid, acetic acid, propionic acid, butyric acid, valeric acid, caproic acid, Structure H-COOH CH3-COOH CH3CH2-COOH CH3(CH2)2-COOH CH3(CH2)3-COOH CH3(CH2)4-COOH IUPAC methanoic acid ethanoic acid propanoic acid butanoic acid pentanoic acid hexanoic acid Positions along the chain are indicated by Greek letters (common names) or numbers (IUPAC). Br O CH3 CHCH2CH2 C OH 5 4 3 2 1 δ γ β α γ-bromovaleric acid 4-bromopentanoic acid Dicarboxylic acids — Structure HOOC-COOH Common Name oxalic acid, IUPAC ethanedioic acid HOOC-CH2-COOH malonic acid, propanedioic acid HOOC-(CH2)2-COOH succinic acid, butanedioic acid HOOC-(CH2)3-COOH glutaric acid, pentanedioic acid HOOC-(CH2)4-COOH adipic acid, hexanedioic acid Aromatic acids — O C O OH benzoic acid C C O C OH O C OH OH O O C OH O isophthalic acid phthalic acid OH C OH terephthalic acid Salts — Name cation followed by acid name with -ic changed to -ate, eg Na+ -O C O 2 sodium benzo ate Physical Properties — Lower molecular weight acids are soluble in H2O: hydrogen bonding. Usually soluble in organic solvents. Sodium and potassium salts of lower molecular weight acids are soluble in H2O and not soluble in organic solvents of low polarity. The salts of longchain carboxylic acids are soaps. Sodium and potassium soaps form micelles in water. A Micelle Carboxylic acids form dimers (pronounced die'-mers) in the liquid phase --O H O R C C R These dimers also O H O exit to some extent in the gas phase, consequently, carboxylic acids have high boiling points for a given molecular weight. 3 Acidity — O O + R C + H 2O R C + H3O O- O H O O R C OO Keq = H3O + R OO Ka = H2O R C + C R H3O C O H O H pKa = -log K a Compare acidity of carboxylic acids with alcohols: CH3CH2OH CH3CH2O- + H+, Ka ~ 10-16 CH3COOH CH3COO- + H+, Ka ~ 10-5 4 Reason — Carboxylate anion is more stable compared to carboxylic acid than alkoxide ion is compared to alcohol. Neither alcohol nor alkoxide ion are stabilized by resonance, but the situation is different for a carboxylic acid O O and its anion; R C R C in this case the O H O H anion is stabilized more important less important more than the acid: O O R R C C O O equally important Substituent groups — Those which stabilize the anion more than its conjugate acid (electron withdrawing) increase acid strength. Those which destabilize anion more than acid (electron donating) decrease acid strength. 5 Synthesis of Carboxylic Acids — Oxidation of Primary Alcohols or Alkylbenzenes – KMnO 4 RCH2OH heat Ar-R [RCHO] KMnO4 heat RCOOH Ar-COOH, where R is 1o or 2o Grignard Synthesis --O O C + R C R O MgX O HX O MgX C R + MgX 2 O H eg + MgBr H3O + CO 2 COOH This method results in a product which has one carbon more than the reactant. 6 Nitrile Hydrolysis — May be carried out under acidic or basic conditions – conditions used may depend on other functional groups present. R C N H2O, H2SO4 heat RCOOH + (NH4)2SO4 H2O, NaOH heat RCOO- Na + + NH3 Mechanism — Acidic Hydrolysis: + R H 3O C N R C N H H2O H R C N H R C HO N H HO H H N H C HO + C N H H2O H2O H R C N H HO H HO R R H2O HO HO H R C R C N H N H H 7 O R C HO H + H N H H Basic Hydrolysis: OH R C OH OH N R H 2O C N R C O N H R C N H H OH O R C O N H OH H R C O + N H OH R H C O + H N H H Aliphatic Nitrile Synthesis — RX + K+CN- DMSO RC N + K+X - Best if R is primary, a disaster if it is tertiary. eg DMSO CH3CH2CH2CH 2Br + CN CH3CH2CH2CH 2CN SN2 but CH3 CH3 DMSO H3C C Br + CN+ HCN H2C C E2 CH3 CH3 8 Reactions of Carboxylic Acids — Reduction to Primary Alcohols — R-COOH (or Ar) 1. LiAlH 4 2. H 3O+ R-CH 2OH In addition to reducing the carboxyl group, LiAlH4 will reduce nitro, nitrile, and various carbonyl groups, among others. If any of these groups are present, they must be protected or another method must be used to reduce the carboxylic acid. 9