Chemistry 20: Calculating Chemical Change Review
advertisement

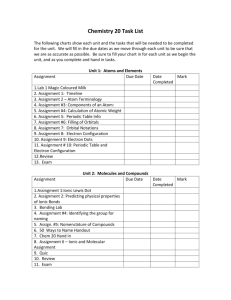
Chemistry 20 – Review Unit Name: __________________________________________________________________ Unit D – Calculating Chemical Change Link of lesson QR code of lesson "Must Know" - I Can… 1 2 3 4 5 6 7 8 9 I can predict the product of a chemical reaction based upon the reaction type. I can recall the balancing of chemical equations in terms of atoms, molecules and moles. I can contrast quantitative and qualitative analysis. I can write balanced ionic and net ionic equations. I can calculate the quantities of reactants and/or products involved in chemical reactions, using gravimetric stoichiometry. I can calculate the quantities of reactants and/or products involved in chemical reactions, using solution stoichiometry. I can calculate the quantities of reactants and/or products involved in chemical reactions, using gas stoichiometry. I can explain chemical principles (conservation of mass) using quantitative analysis. (precipitate calculations) I can identify limiting and excess reagents in chemical reactions. 10 I can define theoretical yields and actual yields. 11 I can calculate % error and explain the discrepancy between theoretical and actual yields. 12 I can draw and interpret titration curves, using data from 13 titration experiments involving strong/weak monoproctic acids and strong/weak monoproctic bases. I can identify equivalence points on titration curves and differentiate between the indicator end point and the equivalence point. Key Vocabulary 1. Qualitative analysis 2. Quantitative analysis 3. Net ionic equation 4. Stoichiometry 5. Gravimetric stoichiometry 6. Gas stoichiometry 7. Solution stoichiometry 8. Limiting reagent 9. Excess reagent 10. Conservation of mass 11. Theoretical yield 12. Actual yield 13. Percentage error 14. Titration 15. Strong/weak Monoproctic acid 16. Strong/weak Monoproctic base 17. Indicator 18. Titrant 19. Equivalence point 20. Buffer zone 21. Endpoint Key equations C = n/V N = m/M Pv = nRt Chemistry 20 – Review Unit Name: __________________________________________________________________