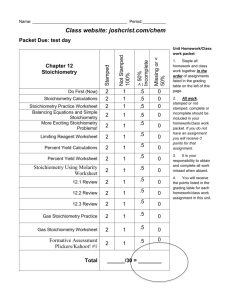
Study Questions Hints: 3. This one is simple just plug and chug a. q
advertisement

Study Questions Hints: 3. This one is simple just plug and chug a. q/change in temp b. q=mCt 5. Find the q 6. This one is just like a station review question # 3 7. Stoichiometry 8. Use energy from # 7 and temp. change calculation. Heat absorbed to water produced. 9. Use info from #7. Find q through stoichiometry then plug into q=mcT 10. Stoichiometry 11. Use each one of these three steps to find Methane using Hess’s law. You have to write the balanced equation for methane first…it is NOT given to you!! 12. Products-Reactants 13. Same as 12 15. find mass first. Q=mcT then stoichiometry