Titration Lesson: Stoichiometry in Chemistry
advertisement
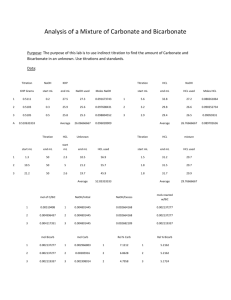
Honors Chemistry Unit 7 – Stoichiometry Lesson 6 – Titrations Book Section: 15-2 Objective: SWBAT use molarity and stoichiometry to solve titration problems. Agenda: Experimental Setup, Lesson, Guided Practice, HW Titration A titration is a lab technique – it is one of the most common quantitative experiments. A titration is a measured reaction between two solutions – one you know the concentration of, and one you don’t. The use of a color-changing indicator tells us when the reaction has reached completion. Solving Titration Problems Titration problems are solution stoichiometry problems – plain and simple. They just refer specifically to the lab technique of titration. Example: A 25 mL solution of 0.5 M NaOH is titrated until neutralized into a 50 mL of HCl. What was the concentration of the HCl? Harder Example Titration reveals that 11.6 mL of 3.0 M sulfuric acid are required to neutralize the sodium hydroxide in 25.00 mL of NaOH solution. What is the molarity of the NaOH solution? You Try When 34.2 mL of a 1.02 M NaOH solution is added from a buret to 25.00 mL of a phosphoric acid solution that contains the indicator phenolphthalein, the solution changes from colorless to red. What is the molarity of the phosphoric acid? HW: #27-30, Read 15-2 Next Week Monday: Stoichiometry Review, Quiz 7-7 Tuesday: Stoichiometry Exam Wednesday: Begin Unit 8: The Periodic Table – History of the Periodic Table (5-1) Thursday: Electron Configurations and the Periodic Table (5-2), Quiz 8-1 Friday: Atomic & Ionic Radius (5-3), Quiz 8-2