Stoichiometry PPT - Chemistry Teaching Resources
advertisement
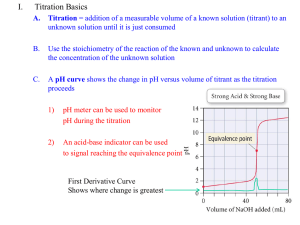
Analysis & Stoichiometry Gordon Watson Chemistry Department, Kelso High School Adv Higher Unit 2 Topic 1 Introduction This topic explores various aspects of Chemical Analysis, leading to an appreciation of the importance of Stoichiometry in chemical reactions. Stoichiometry Stoichiometry involves the understanding of numerical relationships between reacting substances. One methane molecule CH 4 Two oxygen molecules 2 O2 One carbon dioxide molecule CO 2 Two water molecules 2 H 2O The Mole Molar relationships , in turn, allow us to establish measurable relationships between reacting substances. 1 mole moles 16g 32g 1 mole moles 44g 25 l 50 l 25 l 2 2 36g Volumetric Analysis This method of chemical analysis involves accurately measured volumes. Instruments such as pipettes and burettes are used to measure volumes accurately Solutions of unknown concentration are titrated against a solution of known concentration - a primary standard solution or standard solution Gravimetric Analysis This method of analysis involves accurate weighing. Access to an Analytical Balance, capable of reading to 2 decimal places at least, is essential. The analysis will usually involve the production of a suitable precipitate:very low solubility high molecular mass Stoichiometry 1 mole = gfm Primary standard A Primary standard is a substance that has the following characteristics:• a high purity (> 99.9%) • is stable in air and in solution • a reasonably high formula mass • is reasonably soluble Suitable substances include: Potassium hydrogen pthalate (acid) and sodium carbonate Standard Solution Stoichiometry Stoichiometry n = mass / gfm C = n / V Standard Solution A Standard Solution is one whose concentration has been established by titrating against a Primary Standard ….. or against another Standard Solution whose concentration had been established by titrating against a Primary Standard ….. Stoichiometry C1V1 p1 = C2V2 p2 Dilutions Once prepared, standard solutions can be used a stock solutions and further diluted solutions can be made. Stoichiometry C1V1 = C2V2 Titrations Acid-Base Titrations - neutralisation reactions requiring an indicator to detect the end-point NaOH(aq) + CH3COOH(aq) NaCH3COO(aq) + H2O(l) Redox Titrations - based on redox reactions, often self-indicating due to strong colours, e.g. KMnO4 MnO4-(aq) + 8H+(aq) Mn2+(aq) + 4H2O(l) 2I-(aq) I2(aq) + 2e- Complexometric Titrations - based on ligand reactions, requiring an indicator that can be replaced [Ni(In)](aq) + EDTA4-(aq) [Ni(EDTA)]2-(aq) + In Acid-Base Titrations Equivalence point The equivalence point is when the reaction is just completed For a titration between a strong acid (e.g HCl) and a strong base (e.g NaOH) the equivalence point will be when pH = 7. However, not all indicators will complete their colour change at this point so endpoint observed may be different. Indicators Indicators change colour over a pH range. End point In this case both indicators would change just before or just after the equivalence point In this case one indicator would change just after the equivalence point, but the other would be no good. Redox Titrations An excess of MnO4- must be added to detect the end-point. Fortunately MnO4- is so strongly coloured that end-point is very close to equivalence point. Complexometric Titration Murexide indicator forms a yellow2+ green complex with Ni ions. EDTA is added and starts to complex 2+ with any free Ni ions first. Finally, EDTA will replace the murexide molecules and the colour of free murexide - purple - will be produced. Any decision about the end-point relies on there being enough free murexide to produce a distinct colour change. What about the equivalence point? Difficult Titrations The weaker the acid, the smaller the region of rapid pH change which includes the equivalence point. For very weak acids, it is impossible to detect an endpoint close to the equivalence point Back Titration The solution to this problem is a technique known as a back titration. A carefully measured volume of base would be added to ensure complete reaction of the weak acid. A strong acid would then be used to determine the excess base left over. The amount of base which reacted with the weak acid can now be calculated and, hence, the amount of weak acid present originally. Analysis & Stoichiometry End of Topic 1