Science 10 – Chapter 4 Review
advertisement
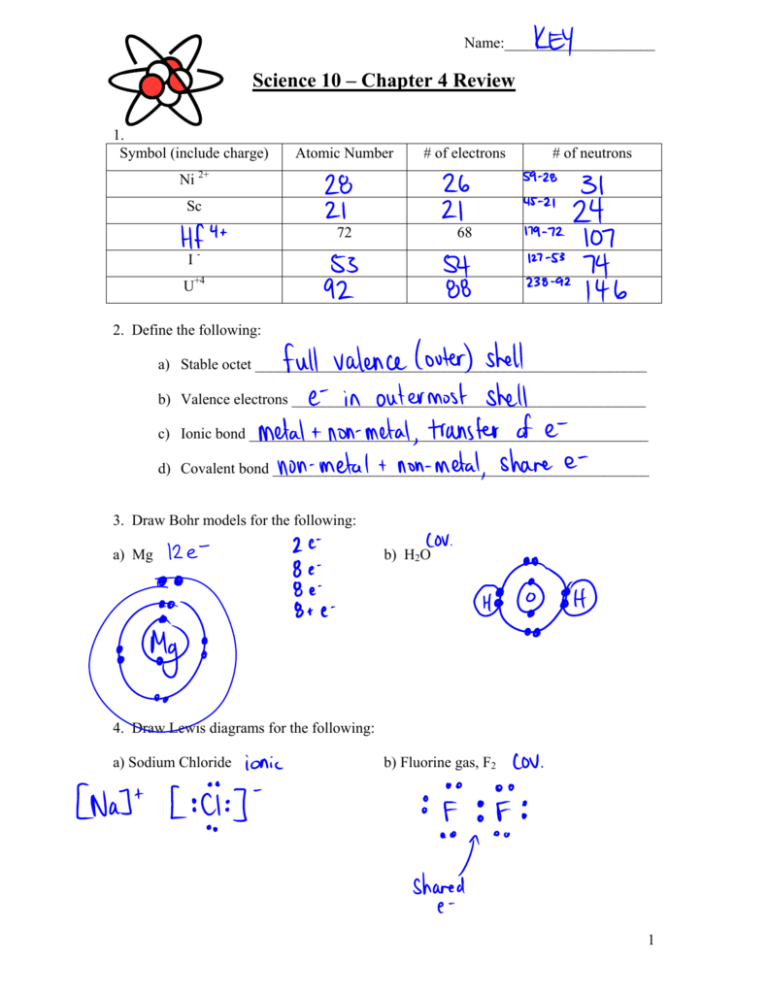
Name:____________________ Science 10 – Chapter 4 Review 1. Symbol (include charge) Atomic Number # of electrons 72 68 # of neutrons Ni 2+ Sc IU+4 2. Define the following: a) Stable octet ____________________________________________________ b) Valence electrons _______________________________________________ c) Ionic bond _____________________________________________________ d) Covalent bond __________________________________________________ 3. Draw Bohr models for the following: a) Mg b) H2O 4. Draw Lewis diagrams for the following: a) Sodium Chloride b) Fluorine gas, F2 1 5. On the following periodic table, identify -a family -a period -halogens -alkali metals noble gases 6. Identify the following atoms (no ion charge) from their Bohr diagrams. 7. When naming a compound with a multivalent metal, what must you include in the name? 2 8. Determine the metal ion and its charge in each of the following compounds. a) CoCO3 ........................The metal ion is _________________ b) Pd(ClO)2 ....................The metal ion is _________________ c) Ru(CO3)2 ...................The metal ion is _________________ d) U(CrO4)3 ...................The metal ion is _________________ 9. Name the following compounds. Include an ‘i’ for ionic and ‘c’ for covalent bonds Si2Br6 CrF2 AuP Be(CN)2 OsPO3 P2S3 N2O3 MnF3 RuN Mg(NO3)2 CuCO3 As2O5 Zr(MnO4)4 HgSO3 Fe(ClO)2 3 10. Write formulas for the following compounds. Include an ‘i’ for ionic bond and ‘c’ for covalent bond Potassium arsenide Calcium selenide Cobalt (III) nitride Beryllium chromate Yttrium hydrogen sulphite Tricarbon pentoxide Silicon tetrachloride Lithium bromide Barium oxide Nickel (III) phosphide Scandium phosphite Radium acetate Dinitrogen monoxide Tricarbon tetraiodide Thallium (III) bisulphate Hexaboron monosilicide Magnesium hydroxide Ammonium dichromate 4 9. An unknown element (we’ll call “Y”) forms the following compounds: Y(NO3)5 Y2(CrO4)3 and Y(MnO4)3 From the information given, what possible ions (with their charges) does Y form? _________________________________________________________________ 9. Balance the following equations. a) Al + b) PbCl4 c) Br2 d) Na2CO3 e) Mn f) C2H6 + g) K2SO4 h) Ca(OH)2 i) Mg3N2 F2 + K3PO4 + + FeI3 + AlF3 KCl I2 Cr(NO3)3 + I2 MnI4 O2 CO2 + AgNO3 + Mg + Pb3(PO4)4 FeBr3 NaNO3 + + Cr2(CO3)3 H2O Ag2SO4 HCl + CaCl2 + + KNO3 H2O N2 10. Write skeleton equations for the following chemical reactions, and balance them. Be sure to check your formulas carefully before you balance! a) lithium phosphate + magnesium sulfate lithium sulfate + magnesium phosphate b) zinc iodide + copper (I) nitrate zinc nitrate + copper (I) iodide c) mercury (II) nitrate + sodium hydrogen carbonate sodium nitrate + mercury (II) hydrogen carbonate 5 d) nickel (III) iodide + iron (II) sulfide nickel (III) sulfide + iron (II) iodide e) aluminum hydroxide + hydrogen fluoride aluminum fluoride + water f) calcium bromide + potassium carbonate calcium carbonate + potassium bromide g) titanium (III) fluoride + cesium sulfite cesium fluoride + titanium (III) sulfite h) barium sulfate + sodium hydroxide sodium sulfate + barium hydroxide i) calcium chloride + potassium potassium chloride + calcium j) tricarbon octahydride + oxygen carbon dioxide + water Remember the diatomic elements! H2O2F2Br2I2N2Cl2 k) sodium + fluorine sodium fluoride 6