Electron configurations and boxes - SCH4U1-CCVI
advertisement

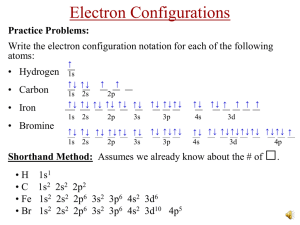
ELECTRON CONFIGURATIONS Orbital energies within a Principle Energy Level of multi electron atoms are split. To create electron configurations or orbital box diagrams for the ground state, to following considerations are applied: Aufbau Principle each additional added electron will occupy the lowest available energy state. Hund’s Rule electrons must be distributed among orbitals of equal energy in such a way that as many electrons as possible remain unpaired. eg. px then py then pz. Pauli Exculsion Principle no 2 electrons within an atom may have the same set of 4 quantum numbers. eg. P [Ne] 3s2 3p3 P [Ne] S [Ne] 3s2 3p4 S [Ne] Ionic and Anomalous Configurations and Magnetism Ion Charges – are formed when: the valence electrons are removed to obtain symmetrical structures, like the noble gases with filled shells. Transition elements can’t do this because of d electrons Symmetry is obtained by having: fully-filled, half-filled and/or empty sub-shells. it is more stable to have a full (or half-full) d sublevel than a full s sublevel eg. Ag [Ar] 4s2 3d9 Ag [Ar] Ag should lose its 2 valence 4s Ag2+ [Ar] 4s0 3d9 Ag2+ [Ar] Actual Ag1+ [Ar] 4s0 3d10 Ag1+ [Ar] Not symmetrical Symmetrical! For metals under the metal/non-metal staircase: eg. Pb [Xe] 6s2 4f14 5d10 6p2 Pb2+ [Xe] 6s2 4f14 5d10 Pb4+ [Xe] 4f14 5d10 inner pair effect Anomalous Configurations occur when the atom is unstable in the ground state – several examples in the transition metals only need to know for 4U , Cr and Cu Cr should be Cr [Ar] 4s2 3d4 Cr [Ar] Actually exists as Cr [Ar] 4s1 3d5 Cr [Ar] Similarly, Cu should be Cu [Ar] 4s2 3d9 Cu [Ar] Actually exists as Cr [Ar] 4s1 3d10 Cr [Ar] Explaining Magnetism Ferromagnetic Elements – Fe, Co, Ni have 4,3,2 unpaired electrons with small, closely packed atoms can form permanent “domains” where all the atoms align their “north poles” in the same direction can exist in the elements and also ionic compounds of these metal ions Paramagnetism due to unpaired electrons in electron configuration can occur in atoms, molecules and ionic compounds “domains” of atoms do not form, but atoms do have a dipole moment and a magnetic field can align these dipoles much less powerful than ferromagnetism