Atomic Model Evolution: Worksheet & Note-Taking Guide
advertisement

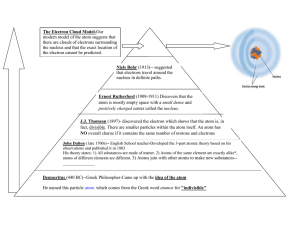
E.Q. All notes must be in the student’s own handwriting. Chunk the information on the right to form questions in this column. Describe how the atom model has progressed through time. Atomos – Greek word for “cannot be cut” or “indivisible”. Atoms are the smallest piece of matter that cannot be commonly broken down. Dalton – Thought all matter was made of particles shaped like simple solid spheres. Matter is made from atoms that can be combined. Atoms of the same element are exactly the same. Atoms of different elements are different. Atoms of different elements can be combined to form new substances. Thomson – discovered electrons and added them to the solid sphere. Rutherford – discovered empty space and the positively charges nucleus in the center. Bohr – concluded that the electrons are orbiting around the nucleus in different energy levels and called them shells. Electron Cloud Model – most recent model, the electrons are moving around the nucleus in an area called the electron cloud. Summary Write a summary about the evolution of the atom model here. Make sure to use complete sentences and write a full paragraph, not just a few sentences.