Irvington High School • AP Chemistry
advertisement

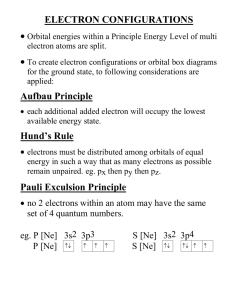
Irvington High School • AP Chemistry Mr. Markic Name _______________________________ Number ___ Date ___/___/___ 7 • Electronic Structure of Atoms ELECTRON CONFIGURATIONS 1. Indicate which of the following sets of quantum numbers in an atom are unacceptable and explain why: a. (1, 0, ½, ½) (a) is wrong because the magnetic quantum number ml can have only whole number values. b. (3, 0, 0, + ½) c. (2, 2, 1, + ½) (c) is wrong because the maximum value of the angular momentum quantum number l is n 1 d. (4, 3, -2, + ½) e. (3, 2, 1, 1) (e) is wrong because the electron spin quantum number ms can have only half- integral values. 2. The atomic number of an element is 73. Is this element diamagnetic or paramagnetic? Since the atomic number is odd, it is mathematically impossible for all the electrons to be paired. There must be at least one that is unpaired. The element would be paramagnetic. 3. Indicate the number of unpaired electrons present in each of the following atoms: a. B: B:[He]2s22p1 (1 unpaired electron) g. Kr: Kr:(0 unpaired electrons) b. Ne: Ne:(0 unpaired electrons, Why?) h. Fe: Fe:[Ar]4s23d6 (4 unpaired electrons) c. P: P:[Ne]3s23p3 (3 unpaired electrons) i. Cd: Cd:[Kr]5s24d10 (0 unpaired electrons) d. Sc: Sc:[Ar]4s23d1 (1 unpaired electron) j. I: I: [Kr]5s24d105p5 (1 unpaired electron) e. Mn: Mn:[Ar]4s23d5 (5 unpaired electrons) k. Pb: Pb:[Xe]6s24f145d106p2 (2 unpaired electrons) f. Se: Se:[Ar]4s23d104p4 (2 unpaired electrons) 4. Page 1 of 2 Write the ground state electron configurations (short-hand) for the following elements: a. B B: 1s22s22p1 b. V V: [Ar]4s23d3 c. Ni Ni: [Ar]4s23d8 d. As As: [Ar]4s23d104p3 e. I I: [Kr]5s24d105p5 f. Au 5. Au: [Xe]6s14f145d10 Use the Aufbau Principle to obtain the ground state electron configuration (short-hand) of selenium. [Ar]4s23d104p4 6. Use the Aufbau principle to obtain the ground-state electron configuration of technetium. The ground state electron configuration of Tc is: [Kr]5s24d5. Page 2 of 2