History of the Atom: Scientists & Models Worksheet
advertisement

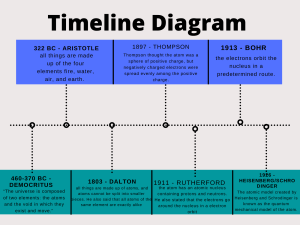
Historical Development of the Atom Explain the contributions made by each of the following in the development of our understanding of the atom. 1. Democritus: first one to state that atoms existed. 2. John Dalton: developed the atomic theory; had a solid sphere as his model—like a billiard ball. 3. J. J. Thomson: discovered the electron; had the plum pudding model—but What does that mean-*think chocolate chip ice cream 4. Ernest Rutherford: performed the gold foil experiment; determined atoms are mostly empty space, have a nucleus which is positively charged and massive 5. Robert Millikan: determined the charge and mass of an electron 6. Neils Bohr: electrons move in orbits around the nucleus—called the Planetary model; stated electrons in atoms have fixed amounts of energy 7. Dmitri Mendeleev: first one to arrange periodic table—done in columns by increasing atomic mass 8. Henry Moseley: arranged periodic table by increasing atomic number 9 Max Planck energy atoms can only change in small fixed amounts known as quanta 10. Werner Heisenberg: developed uncertainty principle- you can know where an electron is or its speed but not both. 11. Louis de Broglie: developed the Quantum mechanical model.