SG key
advertisement

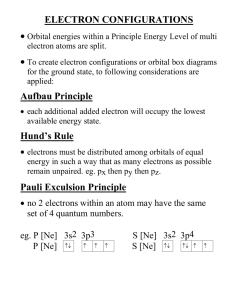
Honors Chem: Ch. 5 and 6 Study Guide-Key Review ALL notes, examples, and worksheets done for this unit!! Everything will be tested. Vocab Anion Electronegativity Isoelectronic Series Principal energy level Atomic orbital Group Pauli exclusion principle Atomic radius Hund’s rule Period Aufbau principle Ion Photoelectric effect Cation Ionization energy Photon Valence electron Be able to: write electron configurations, noble gas electron configurations, draw orbital diagrams, exceptions to normal electron configurations, calculate wavelength, frequency, energy and relate each other. Identify Alkali metals, alkaline earth metals, halogens, noble gases, transition metals, lanthanides, actinides, metals, nonmetals, metalloids Know the properties of metals, nonmetals, and metalloids. The trends in the periodic table for atomic radius, ionic radius, isoelectronic series, electronegativity, ionization energy, and compare atoms vs. ion size for cations and anions. Make sure you know WHY and the EXCEPTIONS!!! Ch. 5 Pg. 146-148 32. light, microwaves, x rays, radio waves 41.the quantum number n specifies the electron’s orbit. 49. b, c are incorrect 52. two electrons 59. Two electrons occupying a single atomic orbital must have opposite spins. 61.Valence electrons are the electrons in the atom’s outermost orbital; 2 70. E= 2.97 x 10 -19 J 72. E= 1.68 x 10 -17 J 79 a. Mn: [Ar] 4s23d5 b. Kr: [Ar] 4s23d104p6 c. P: [Ne]3s23p3 d. Zn: [Ar]4s23d10 e. Zr: [Kr]5s24d2 80 a. F b. Ca c. Nd d.Te e. Md f. Br 82. 18; 15; 4 Ch. 6 Pg. 165 16. Largest: Na, smallest: s 17. Largest: Xe, smallest: He Pg. 174-176 33 a. 2 b.4 c. 3 d.1 36. properties describe a metal; left of the stair step line 40 a. Br, Hg b. Rn c. Sn or Pb d. elements 58-71 or 90-103 42. The number of valence electrons equals the group number for group A elements. 44. All noble gases have eight valence electrons, except helium, which has 2. 50 a. Bi: [Xe] 6s2 4f14 5d10 6p3 b. Cl: [Ne] 3s2 3p5 c. Li: [He] 2s1 d. Hg: [Xe] 6s2 4f14 5d10 51.because the boundaries of an atom are indistinct 55.Elements on the right side of the periodic table gain electron to gain a stable octet. 59. The group 8A elements have the highest ionization energies because their electron configurations are the most stable. 63.a. N b. Ne C. Li 65 a. the ion is negative. A negative ion is always larger than its own atom. b. A is to the left of B. Atomic radius in a period decreases from left-to-right c. A is below B. Ionic radius increases down a group. 66 a.8 b.3 c.1 76. The element is most likely a nonmetal.