Document
advertisement

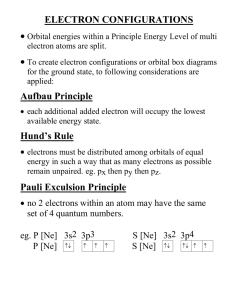
Bonding Unit Review Guide 1. Write the full electron configuration for the following atoms or ions and determine the number of unpaired electrons: a. Cl ______________________________________________ ____ unpaired electrons b. Kr ______________________________________________ ____ unpaired electrons c. Mg+2 ___________________________________________ ____ unpaired electrons 2. Write the abbreviated electron configurations for the following atoms or ions and determine the number of unpaired electrons: a. Pb ______________________________________________ ____ unpaired electrons b. Cs ______________________________________________ ____ unpaired electrons c. Br-1 _____________________________________________ ____ unpaired electrons 3. What is Hund’s Rule? Pauli Exclusion Principle? Aufbau Principle? 4. Draw all the possible Lewis Structures for NO3-1 and discuss the bond orders, bond lengths, and bond strengths. 5. What are the exceptions to the octet rule and for what atoms do they apply to? 6. Determine the enthalpy change for the following reactions using bond dissociation energies from your table: a. CH2Cl2 + Cl2 CCl4 + H2 b. N2 + 3Cl2 2NCl3 c. CH4 + 2O2 CO2 + 2H2O 7. Name SO3-2 NO2-1 ICl3 HClO3 Lewis Structure Molecular Geometry Bond Order Longest Bond? Show the dipoles Are the bonds polar or nonpolar? How much energy is needed to break up the molecule? Give the bond angle (or an approx) for each bond in the molecule 8. For each of the following, answer the question then explain your reasoning. a) Which has the lower electron affinity…Cl or Ca b) Which has a higher ionization energy…Be or Mg? c) Which has the lower ionization energy…Ag or I? d) Which has a higher electron affinity…Na or Kr? 9. Place the following in order of increasing size then explain your reasoning: Fe+2, Sn+2, Sn+4, Fe+1 10. Place the following in order of increasing ionization energy then explain your reasoning: K, Ca+2, P, K+1