chemical bonding theory 2-Q
advertisement

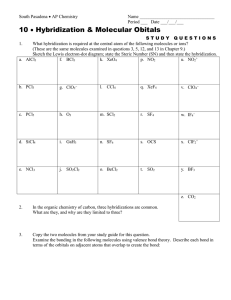
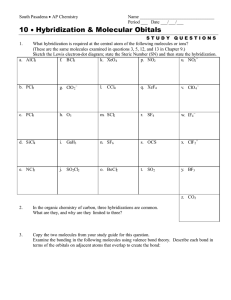
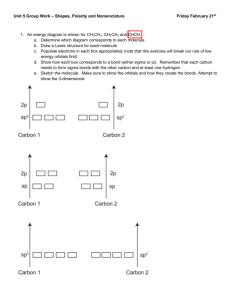
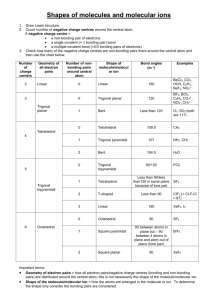
CHEM 105 (Chemical Bonding)- Problem Set 2 1. Describe the hybridization scheme for the central Cl atom in the molecule ClF3. What orbitals of the Cl atom are involved in overlap, and which are occupied by lone-pair electrons? 2. Use the method of Figure 12-16 to represent bonding in each of the following molecules: a) CCl4; b) ONCl; c) HONO; d) COCl2. 3. Draw the hybrid orbital of the molecule allene (CH2CCH2). Make sure to designate the hybridization of carbon atoms. 4. N2(g) has exceptionally high bond energy. Would you expect either N2- or N22- to be a stable diatomic species in the gaseous state? Explain. 5. Assume that the energy-level diagrams of for diatomic molecules apply to diatomic ions CO+ and CN-. a) Predict the bond order of each. b) Which of these ions is paramagnetic? diamagnetic? c) Which of these ions do you think has the greater bond length? Explain. 6. Draw the Lewis structure for the Urea molecule CO(NH2)2, and predict its geometric shape with the VSEPR theory. Then revise your assessment of this molecule, given the fact that all the atoms are 120o. Propose a hybridization and bonding scheme consistent with these experimental observations. 7. Draw a Lewis structure(s) for the nitrite ion, NO2-. Then propose a bonding scheme to describe the s and p bonding in this ion. What conclusions can you reach about the number and types of p molecular orbitals in this ion? Explain. 8. The molecule formamide, HCONH2, has the following approximate bond angles: H-C-O, 123o; H-C-N, 113o; N-C-O, 124o; C-N-H, 1190; H-N-H, 119o. The C-N bond length is 138pm. Two Lewis structures can be written for this molecule, with the true structure being a resonance hybrid of the two. Propose hybridization and bonding scheme for each structure. 9. The ion F2Cl- is linear, but the ion F2Cl+ is bent. Describe hybridization scheme for the central Cl atom consistent with the difference in structure.