Lewis Structure/Molecular Model Lab Questions
advertisement

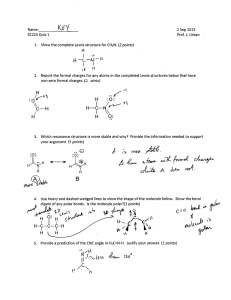
Questions for Lewis Structure/Molecular Model Lab Use complete sentences to answer. 1. What was the purpose of this lab? 2. Explain why a tetrahedron molecule containing four identical atoms attach to central atom can never be a polar molecule. 3. How can a non-polar trigonal planar molecule become a polar molecule without changing the central atom? 4. Why is trigonal pyramid molecule always polar? 5. Briefly explain the two ways you can determine if two atoms will join using a covalent bond. 6. When drawing the Lewis Structure for a negative ion, what must you do? for a positive ion? 7. How do you determine, when looking at a Lewis Structure, if the electrons are being shared equally or unequally? 8. Create Lewis Structures for the five geometric shapes covered in class in the space below. However, you may not use any molecule seen in your notes or lab. Be certain to label each drawing with its appropriate shape.