Quiz 444
advertisement
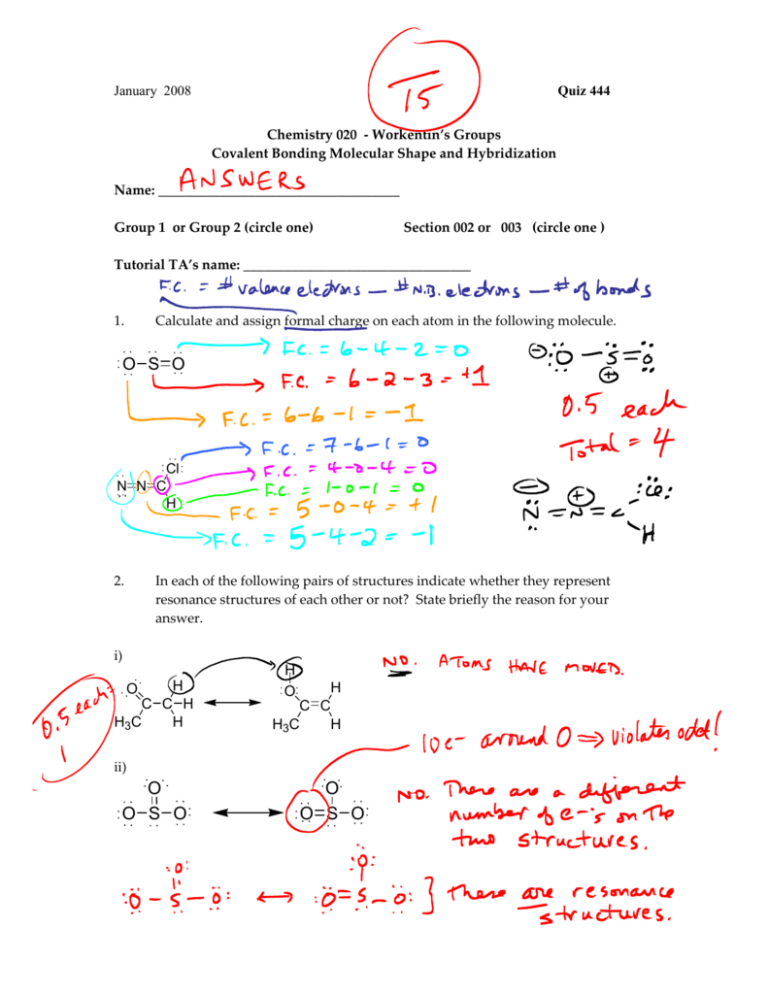
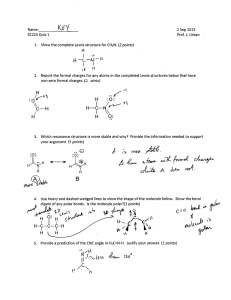
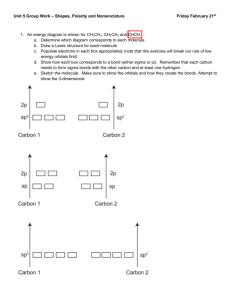
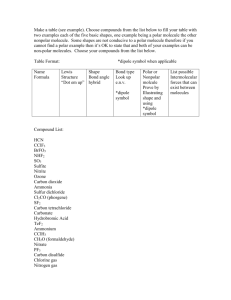
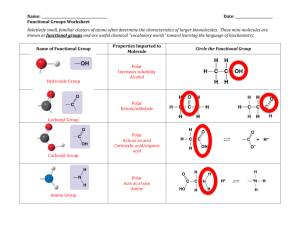
January 2008 Quiz 444 Chemistry 020 ‐ Workentin’s Groups Covalent Bonding Molecular Shape and Hybridization Name: ___________________________________ Group 1 or Group 2 (circle one) Section 002 or 003 (circle one ) Tutorial TA’s name: _________________________________ 1. Calculate and assign formal charge on each atom in the following molecule. O S O Cl N N C H 2. In each of the following pairs of structures indicate whether they represent resonance structures of each other or not? State briefly the reason for your answer. i) H H H O O C C H C C H3C H H3C H ii) O O S O O O S O January 2008 3. Quiz 444 For each of the following: i. Draw the appropriate Lewis structure ii. Write the shape of the molecule iii. State the hybridization at the central atom (sp, sp2, sp3, sp3d, or sp3d2) iv. State whether the molecule is polar or non‐polar Formula Lewis Structure Shape Hybridization Polar or non‐polar H2Se SCl3+ SiF5 XeOF4 IF6+