Chem I Worksheet week 12
advertisement

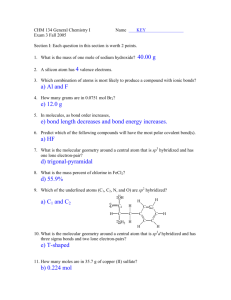
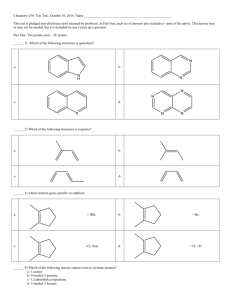
Center for Academic Services & Advising November 20, 2015 Chemistry I CSI Worksheet 11 1. A molecule with the formula AB3 has a trigonal planar geometry. How many electron groups are on the central atom? 2. Determine the electron geometry, molecular geometry, and idealized bond angles for each molecule. In which cases do you expect deviations from the idealized bond angle? a. CF4 b. NF3 c. OF2 d. H2S 3. Determine if the above molecules are polar or non-polar. 4. Which species has a smaller bond angle, ClO4- or ClO3-? 5. CH3F is a polar molecule despite tetrahedral geometry often leading to non-polar molecules. Why? COLORADO SCHOOL OF MINES Frances I Core Supplemental Instruction Facilitator | Chemistry II Center for Academic Services & Advising 6. The valence bond configuration of several atoms are shown. How many bonds can each atom make without hybridization? a. B: 2s22p1 b. N: 2s22p3 c. C: 2s22p4 7. Which of the following hybridization schemes allow the central atom to form more than four bonds? a. sp3 b. sp3d c. sp2 8. Draw an energy diagram for HCl. Predict the bond order and make a sketch of the lowest energy bonding molecular orbital COLORADO SCHOOL OF MINES Frances I Core Supplemental Instruction Facilitator | Chemistry II