Molecular Modeling Lab Activity
advertisement

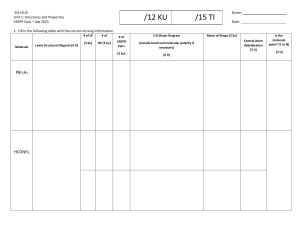
Molecular Modeling Lab Activity Instructions: 1. Draw all Lewis Structures first. Check the key to ensure that your structures are correct. If any of your structures are incorrect, reevaluate or ask questions. 2. Fill in the steric number, bonding atoms, and lone pairs on your Lewis structures. 3. Use the information from number 2 to determine the electron pair arrangement and molecular shape. This requires knowledge of VSEPR theory. Use your handout. 4. Build your molecule using the molecular modeling kit supplied to you. 5. Draw the best 3-D representation of your molecule in the space provided. 6. Analyze the shape and commit the 3-D image to memory. You will not have the models when taking quizzes or tests. 7. Based on the shape and bonding atoms determine whether your molecule is polar or nonpolar. 8. Turn in your completed sheet and return the models to the side shelf.