Molecular Structure Worksheet: Hybridization & Shapes
advertisement

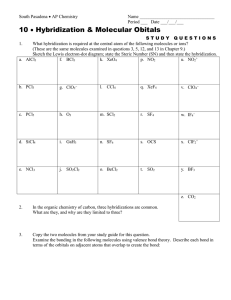
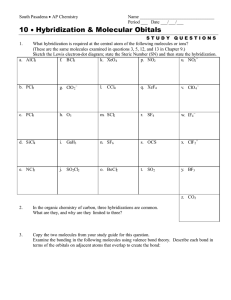
Molecular Structure Worksheet 2 1. Phosphorus reacts with chlorine to form two compounds PCl 3 and PCl5. What is the hybridization and shape for each molecule? 2. When nitrogen reacts with chlorine only ONE compound (NCl 3) is formed. Explain why nitrogen is different from phosphorus in this reaction. 3. What are the approximate bond angles in each of the following? Justify your answers. (A) ONO in NO 3(B) HPH in PH 3 (C) FSF in SF 6 4. What is the shape and hybridization of the central atom for each of the following? (A) SO 3 (B) XeF 2 (C) IF 5 (D) SeF 6 (E) ICl 4(F) I 35. Draw a Lewis electron-dot structure for each of the molecules below and identify the shape of each. XeF 4 ClF 3 CF 4 (i) Identify the hybridization of each central atom. (ii) Use the valence shell electron pair repulsion (VSEPR) model to explain the geometry of each of these molecules. 6. The bond angles for H-N-H in the ammonia molecule is 107 °, whereas the bond angle for H-P-H in the molecule PH3 is 93°. Explain: (A) Why the H-N-H bond angle is 107 °. (B) Why the H-P-H bond angle is 93 °. (C) Since N and P are in the same group in the periodic table, why are their bond angles different? 7. Draw a Lewis structure and predict the molecular geometry of each of the following: (A) SiF 4 (B) SeF 4 (C) KrF 4 Explain why these three molecules have different shapes and which, if any, are polar? ©1994–PTAS, Inc. Page 1 of 1