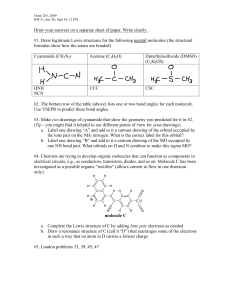
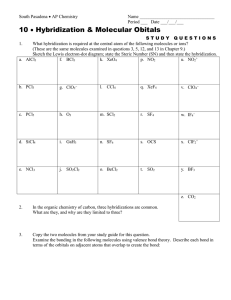
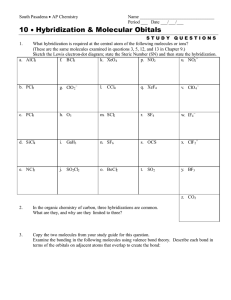
molecular geometry worksheet
advertisement
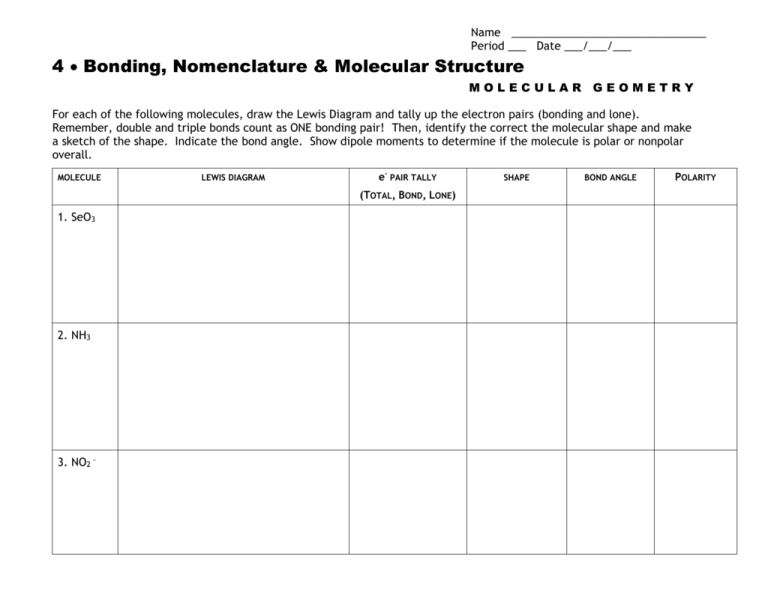
Name ________________________________ Period ___ Date ___/___/___ 4 Bonding, Nomenclature & Molecular Structure MOLECULAR GEOMETRY For each of the following molecules, draw the Lewis Diagram and tally up the electron pairs (bonding and lone). Remember, double and triple bonds count as ONE bonding pair! Then, identify the correct the molecular shape and make a sketch of the shape. Indicate the bond angle. Show dipole moments to determine if the molecule is polar or nonpolar overall. MOLECULE LEWIS DIAGRAM e- PAIR TALLY (TOTAL, BOND, LONE) 1. SeO3 2. NH3 3. NO2 - SHAPE BOND ANGLE POLARITY MOLECULE LEWIS DIAGRAM e- PAIR TALLY (TOTAL, BOND, LONE) 4. BeF2 5. SiH4 6. SeH2 SHAPE BOND ANGLE POLARITY