Covalent Molecular Compounds
advertisement

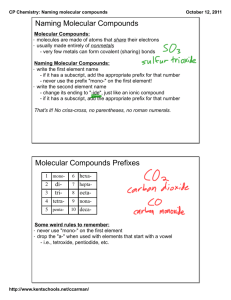
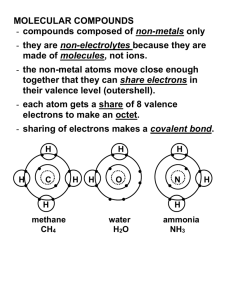
Covalent Molecular Compounds Objectives: • Write chemical formulas for covalent molecular compounds • Name covalent molecular compounds What is a Molecular Compound • Compounds that are formed from 2 non-metals NOTE • Sets of non-metals can form more than 1 compound so we and O canmethod form of need C a different CO or CO 2 naming writing formulas and compounds Prefix System • Valence Numbers are not used • A prefix tells how many atoms of each element are in the molecule • The prefix is NOT crossed • The second non-metal ends in “ide” The Prefixes EXAMPLE • Carbon Dioxide NOTE: Mono is used only on the second non-metal if necessary CO2 EXAMPLE • Carbon Monoxide NOTE: Mono is used only on the second non-metal if necessary CO EXAMPLE • Phosphorus Trichloride PCl3 EXAMPLE • Sulfur Hexafluoride SF6 EXAMPLE • Dinitrogen Monoxide N 2O Naming Covalent Molecular Compounds • Name the prefix for the FIRST subscript Mono is only used • Name first element for the second • Name the prefix for the non-metal SECOND subscript • Name second element • Add “ide” • OF2 EXAMPLE Oxygen Difluoride • Cl2O8 EXAMPLE Dichlorine Octoxide • S2Cl2 EXAMPLE Disulfur Dichloride • CBr4 EXAMPLE Carbon Tetrabromide • P2O3 EXAMPLE Diphosphorus Trioxide Page 167 #64, 66