Covalent Compounds - Salem Community Schools
advertisement

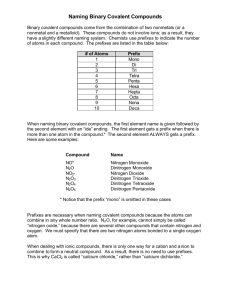
Covalent Compounds This program will help you learn how to name covalently bonded compounds and how to write their formulas. Covalent compounds are formed when two nonmetals share electrons. Where are the nonmetals on the periodic table? That is correct, the nonmetals are on the right side of the periodic table. When naming covalent compounds, you list the name of the most metallic element first. The most metallic would be the element furthest to the left. Since electrons are shared and not lost or gained, we have to have a special method to name these molecules. For this we use PREFIXES. For example, here are two common gases you would find in our air: CO – this gas is a poisonous product of car exhaust. CO2 – this gas is exhaled by humans and plants love it. Can we call both of them carbon oxide? NO, we don’t want to mix them up, it could be deadly. PREFIXES For naming covalent compounds, use the following: 1 - mono 2 - di 3 - tri Exception: For some reason, the prefix mono Is not used 4 - tetra on the first element. 5 - penta 6 - hexa 7 - hepta Only use prefixes with 8 - octa covalent compounds. 9 - nona 10 - deca CO or CO2? Using our prefixes, we now know that…… CO is carbon monoxide CO2 is carbon dioxide Notice the carbon does not have a prefix, mono is not used on the first element. The prefix before oxide indicates the number of oxygen atoms in the compound. N2O3 What would you call it? Dinitrogen trioxide Dinitrogen because there are two nitrogen atoms. Trioxide because there are three oxygen atoms. PH3 What would you call it? Phosphorous trihydride. No mono on the first element. Hydrogen is sometimes a nonmetal, when it is listed last in compound.