Naming Molecular Compounds
advertisement

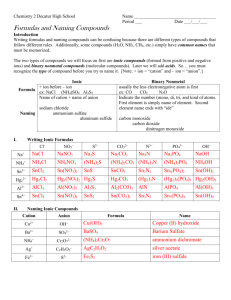
Chem 155 - Eric Bullock Santa Barbara City College Naming Molecular Compounds Molecular compounds make up the vast majority of the millions of known compounds. They consist of nonmetal elements only. Many molecular compounds are very large and complicated such as proteins and DNA. However, we will limit our naming to simple binary molecular compounds that consist of only two different nonmetal elements. The names of these molecules consist of just two words (for example: carbon dioxide). All atoms in all molecules are electrically neutral. When you see an oxygen atom in the chemical formula for a molecule, for example, it is not the O2- ion and does not have an electrical charge of -2. It has an electrical charge of 0. All molecules as a whole are electrically neutral as well. They all have an electrical charge of 0. 1) Naming molecular compounds from their chemical formulas a) The first word in the name of a binary molecular compound is the name of the first element in its chemical formula preceded by a Greek prefix. This Greek prefix is the number of atoms of that element in the molecule. The exception is when there is only one atom of the first element in the formula. In this case no prefix is used. Some examples are given below: The first word in N2O is dinitrogen The first word in NO2 is nitrogen The first word in P3S4 is triphosphorus The first word in SF6 is sulfur (or sulphur) The Greek prefixes are: Number of atoms 1 2 3 4 5 6 7 8 9 10 Greek prefix mono di tri tetra penta hexa hepta octa nona deca b) The second word of a binary molecular compound also uses a Greek prefix to indicate the number of atoms of the second element in the molecule’s chemical formula. After the prefix, the stem of the name of the element is written, followed by the suffix -ide. Completing the names of the examples above: N2O is dinitrogen monoxide NO2 is nitrogen dioxide P3S4 is triphosphorus tetrasulfide SF6 is sulfur hexafluoride from: mono - ox - ide here one ‘o’ is dropped from: di - ox - ide from: tetra - sulf - ide from: hexa - fluor - ide 1 Chem 155 - Eric Bullock Santa Barbara City College 2) Binary molecular compounds that begin with hydrogen Group 6A and 7A in the Periodic Table form the following binary molecular compounds with H: Group 6A H2 O H2 S H2Se H2Te Name water hydrogen sulfide hydrogen selenide hydrogen telluride Group 7A HF HCl HBr HI Name hydrogen fluoride hydrogen chloride hydrogen bromide hydrogen iodide The naming of these compounds is very similar to the naming of other binary molecular compounds except that Greek prefixes are not used. 3) Common names Some molecules are so common that we do not use their chemical names. Instead, we use their common names. We never use the chemical names for these molecules. In this course, there are two molecules for which we use common names: H2 O NH3 water ammonia 4) Elemental molecules Some elements exist in their pure forms as molecules. Because they consist of pure elements, they are not compounds. Here is a table of all the elemental molecules. Chemical Formula Name H2 hydrogen N2 nitrogen O2 oxygen F2 fluorine Cl2 chlorine Br2 bromine I2 iodine P4 phosphorus S8 sulfur Notice that when we use the name of one of these elements such as ‘fluorine’, this can mean either: 1) F, an atom of fluorine 2) F2, a molecule of the element fluorine 2