Molecular Compounds
advertisement

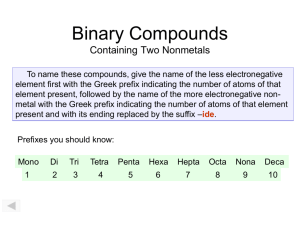
Molecular Compounds Writing Names and Formulas Molecular Compounds Molecular compounds are made of molecules. They are made by joining nonmetal atoms together into molecules. Naming is Easier A molecular compound’s name tells you the number of atoms through the use of prefixes. Prefixes 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca Prefixes The name will consist of two words. Prefix name prefix name -ide One exception is we don’t write mono- if there is only one of the first element. Prefixes The following double vowels cannot be used when writing names: (oa) (oo) Example NO2 There is one nitrogen mononitrogen But, you cannot use mono- on the first element, so drop the prefix. nitrogen mononitrogen Example NO2 There are two oxygens dioxygen Example NO2 dioxygen You need the suffix -ide diox ygen ide Example NO2 nitrogen dioxide Example N2O There are two nitrogens dinitrogen There is one oxygen monooxygen Example N2O monooxygen You cannot run (oo) together, so monoxygen Example N2O monoxygen You need the suffix -ide monoxygen ide Example N2O dinitrogen monoxide Problem Name the following molecular compounds. Cl2O7 CBr4 dichlorine heptoxide carbon tetrabromide Problem Name the following molecular compounds. CO2 carbon dioxide BCl3 boron trichloride Naming Molecular Compounds You will not need to criss-cross oxidation numbers. Molecular compounds name tells you the number of atoms through the use of prefixes. Example diphosphorus pentoxide The name implies there are 2 phosphorous atoms and 5 oxygens. P2O5 Example sulfur hexaflouride The name implies there is 1 sulfur atom and 6 fluorines. SF6 Problem Write the formulas for the following molecules. tetraiodide nonoxide nitrogen trioxide I4O9 NO3 Problem Write the formulas for the following molecules. carbon tetrahydride phosphorus trifluoride CH4 PF3