“Organic Chemistry” (A
advertisement

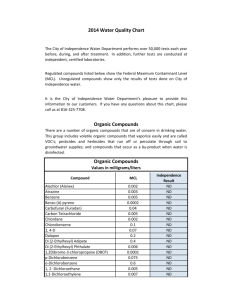
“Organic Chemistry” (A-L) – Natural Science Lecturer: Dott. Stefano Di Stefano Dipartimento di Chimica, floor I, room 191 tel 0649913057 e-mail stefano.distefano@uniroma1.it Students target: 60 Level: introductive Knowledge already acquired: Basic knowledge of general and inorganic chemistry Credits: 6 Contents Subject of the Organic Chemistry, diffusion of organic compounds, chemical formulae (brutal, Lewis, Kekulè), meaning of bond line. Molecular structure, atomic orbitals, ibridization. Reminds on chemical bond, bond length, angle and energy, dipolar moment. Delocalized bonds and resonance. Brønsted acidity and basicità, Ka and pKa, equilibria, acids on oxygen (carboxylic acids, phenols and alcohols), and acids on carbon. Lewis acids. Saturated hydrocarbons: classification, isomerism, names (IUPAC and historical). Alkyl radicals, ramified hydrocarbons, and cycloalkanes. Structure formulae and projective formulae. Molecular models. Ethane, propane, butane conformations, Newman formulae. Conformation analysis of cyclohexane. Substituted cyclohexane and conformational energy of substituents. Trans and cis isomerism. Physical properties: boiling point, solubility, density. Intermolecular forces: van der Waals, dipolar interactions, hydrogen bonding. Importance and presence of alkanes and cycloalkanes in nature. Reactivity: radical reactions: alogenation and auto-oxidation. Combustion reaction. Alkenes and alkynes: names, cis-trans isomerism, E and Z notation, physical properties, importance. Transition state theory (essential concepts). Mechanisms of organic reactions, energy diagrams, intermediates, reaction rate and reaction order, catalysts. General mechanism for electrophylic addition to alkenes. Addition of HX: Markownikoff rule and carbocation stability. Addition of molecular bromine. Hydratation reaction. Catalytic hydrogenation, and alkenes stability. Oxidation with OsO4. Optical stereoisomerism: symmetry and chirality, stereocenters, polarimetry and specific rotatory power, enantiomers, racemic mixture, Fischer projective formulae, relative configuration (D,L), absolute configuration (R, S), Chan-IngoldPrelog priority rules. Prochirality. Compounds with more than one stereocenter: diastereoisomers, mesoforms, resolution of racemic mixtures (mechanical, chemical, biochemical). Halogenated compounds: names and importance, nucleophilic substitution to saturated carbon. SN1 and SN2 mechanisms: stereochemistry, steric effects, nucleophile effects, solvent effects. -elimination reactions, E1 and E2 mechanisms Saytzeff rule. Competition between substitutions and eliminations. Alcohol, phenols, ethers, thiols, sulfides: names, importance and physical properties. Acid properties, Transformation of alcohols into alcohoxides and bromide or chloride. Dishydratation and oxidation of alcohols. Glicols. Transformation of alkenes into glicols: sterechemistry. Arenes: names subtitution in benzene, polycyclic and eterocyclic aromatic compounds. Huckel rule. Electrophylic aromatic substitution: general mechanism. Halogenation, nitration, sulfonation, Friedel-Crafts alkylation and acylation. Activations and orientation concepts. Amines: names, importance. Basicity of aliphatic and aromatic amines. Carbonylic compounds, aldheydes and ketones, names, importance, physical properties. Nucleophylic additions. Acetals and ketals formation. Schiff bases. Reductions and oxidations of carbonylic compounds. Prototropic tautomerism: enoles and enolates. Aldolic reaction and condensation. Carboxylic acids: names, importance and physical properties. Conversion to chlorides, bromides, esters, amides, anhydrides. Esters; names importance and physical properties. Basic and acid hydrolysis: mechanisms. Lipids and fat acids. Surfactants, Micelles, Phopholipids, lipidic bilayers. Carbohydrates: classification, and importance. Aldoses and ketoses: Fischer and Haworth formulae, conformational representations. Anomers, epimers. D-glucose, D-mannose, D-fruttose, D-ribose. Glicosides. Disaccharides: saccharose, maltose. Polisaccharides. Ploipeptides and proteins. Natural aminoacids: structure and chimico-physical properties. Isoelectric point. Primari, secondary, tertiary and quaternari structures of proteins. Nucleosides and nucleotides, nucleic acids. Importance of the bases. DNA and RNA structures. Genetic code and biosynthesis of proteins. Expected output from the students The student is expected to be prepared on the main general subjects of organic chemistry, from the molecular structures of the most important organic molecules to their reactivity. Furthermore the student is requested to be competent to discuss the basic concepì of biochemistry. CONTENTS Introduction Saturated hydrocarbons Unsaturated hydrocarbons Stereoisomerism Aromatic compounds Monofunctional compounds Biomolecules lectures Practice lectures Practice lectures Practice lectures Practice lectures Practice lectures Practice lectures Lecture hours Study hours Total hours 6 2 2 1 4 1 7 2 3 1 10 4 8 51 13 3 4 1 8 2 16 3 6 1 20 4 18 99 19 5 6 2 12 3 23 5 9 2 30 8 16 150 Evaluations Final evaluation The evaluation is based on a written examination followed by an oral one.. Suggested Books W. H. Brown, T. Poon “INTRODUZIONE ALLA CHIMICA ORGANICA” Third edition, EdiSES, Napoli 2005 oppure: H. Hart, L. E. Craine, D. J. Hart, C. M. Hadad “CHIMICA ORGANICA” Sixth edition, Zanichelli, Bologna 2008 P. Y. Bruice “ELEMENTI DI CHIMICA ORGANICA” First Edition, EdiSES, Napoli 2007 J. McMurry “FONDAMENTI DI CHIMICA ORGANICA” Third edition, Zanichelli, Bologna 2005