WS Naming Covalent Compounds
advertisement

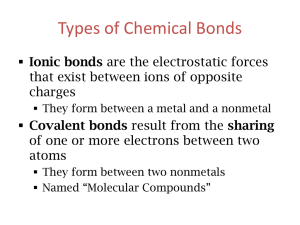
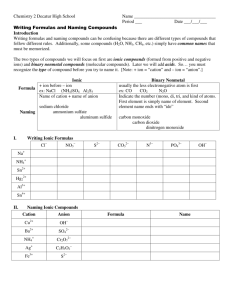
Name:___________________________________ Date:____________ Period:_____ WS Naming Covalent (Molecular) Compounds Rules for naming binary molecular compounds 1st element has normal element name. 2nd element has ending changed to –ide. Use prefixes to indicate how many of each element. Only use mono- with a single oxygen as 2nd element. 1 mono 6 hexa Molecular Naming Prefixes 2 di 3 tri 4 tetra 7 hepta 8 octa 9 nona 5 penta 10 deca A) Write Names for these Molecular Compounds. Formula Name 1. Cl2O7 _______________________________ 2. N2O5 _______________________________ 3. P2Cl3 _______________________________ 4. ClO4 _______________________________ 5. SBr2 _______________________________ 6. SF6 _______________________________ 7. PCl3 _______________________________ 8. S2F10 _______________________________ 9. N2O _______________________________ 10. NF3 _______________________________ 11. SeF6 _______________________________ 12. Si2Br6 _______________________________ 13. CCl4 _______________________________ 14. CO _______________________________ 15. P2O5 _______________________________ 16. NF3 _______________________________ 17. P4S5 _______________________________ 18. SO2 _______________________________ 19. XeF4 _______________________________ 20. SiH4 _______________________________ OVER 1 B) Write Formulas for these Molecular Compounds. Name Formula 1. dinitrogen tetrahydride ____________ 2. carbon tetrachloride ____________ 3. diphosphorus trioxide ____________ 4. dichlorine heptoxide ____________ 5. carbon dioxide ____________ 6. carbon disulfide ____________ 7. iodine monofluoride ____________ 8. nitrogen triiodide ____________ 9. silicon disulfide ____________ 10. dinitrogen tetrabromide ____________ 11. arsenic tribromide ____________ 12. hexaboron silicide ____________ 13. chlorine dioxide ____________ 14. hydrogen iodide ____________ 15. iodine pentafluoride ____________ 16. dinitrogen trioxide ____________ 17. ammonia ____________ 18. phosphorus triiodide ____________ 19. tetraphosphorus decoxide ____________ 20. dihydrogen monoxide ____________ 2