File
advertisement

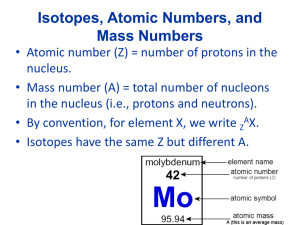
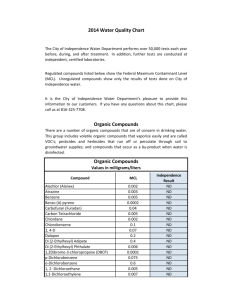
Matter Substance (Homogeneous= same throughout, uniformly composed) Element -Can not be broken down by any means -Only ONE capital Letter Ex -Na Mixtures combined and broken down physically Compound -two of more elements Combined Chemically in a definite ratio - Two or more capital letters Ex- NaCl Homogeneous (same throughout) - Solutions - Something dissolved into H2O is Aqueous Ex- NaCl(aq)= salt water Heterogeneous (different throughout) - Garbage -Soil Diatomics- a group of elements that exist in pairs (theirs 7 of them!) H2 = Hydrogen O2= Oxygen F2= Fluorine Br2= Bromine I2= Iodine N2= Nitrogen Cl2= Chorine Particle Diagrams -Monatomic elements -Diatomic Elements -Compound -Mixture of elements -Mixture of compounds -Mixture of elements AND compounds Formula Writing Ionic Compounds- A transfer of electrons from a metal to a non-metal Caton= positively charged ion (Metal of NH3) Anion= negatively charged particle (-Non-metal or polyatomic ion Table E) Covalent (Molecular)- a sharing of electrons between non-metals Both qualitative and quantitative information is given in a chemical formula Qualitative= what elements are Present Quantitative= how many atoms of each element are present Ex- H2OQualitative = Hydrogen, Oxygen Quantitative = 2=Hydrogen 1= Oxygen Counting Atoms Coefficients represent Moles 2H2O= two moles of water Hydrogen = 4 atoms Oxygen = 2 atoms If theirs Multiple polyatomic ions in a compound use parentheses Empirical Formula - Represents the simplest whole number ratio of atoms in a compound -Ionic compounds are always written in empirical form - Covalent compounds can have both a molecular form and an empirical form - Molecular formula = represents the actual number of atoms in a molecule Empirical ex- C6H12O6 CH6O Naming Covalent Compounds Binary compound = a compound composed of two elements All binary compounds end in the “ide” suffix 1st element = 1 atom = NO prefix 2 or more = prefix 2nd element always gets a prefix and ends in “ide” Prefix Mono = 1 Di = 2 Tri = 3 Tetra = 4 Penta = 5 Hexa = 6 Hepta = 7 Oct = 8 Non = 9 Deca = 10 CO2 N2O5 Carbon Dioxide P4O10 Dinitrogen Pentoxide Tetraphosphorus decoxide Naming Binary Ionic Compounds (with 1 oxidation state) 1. CHECK for a metal 1 oxidation name the metal as is and add “ide” suffix to the Non-metal 2. CHECK the number of oxidation states More than one oxidation state use the stock system NaCl Sodium Chloride AlBr3 Aluminum Bromide Formula Writing with Binary Ionic Compounds 1. Crisscross the oxidation state to become subscripts 2. Reduce the ratio of atoms Ex- Sodium Bromide Na +1 Br Sodium Oxide Na+1O-2 -1 NaBr Na2O Formula Writing- Ionic Compound containing polyatomic ions (TABLE E) Sodium Carbonate Aluminum Carbonate Na+1(CO3)-2 Al+3(CO3)3 Na2CO3 Al2(CO3)3 The Stock System The stock system is used when naming and writing formulas from a compound in which one of the elements take on multiple oxidation states. I = +1 II = +2 III = +3 IV = +4 V = +5 VI = +6 VII = +7 Iron (II) Oxide Tin (II) Sulfide Fe+2O-2 Sn+2S-2 FeO SnS Naming Ionic Compounds Using the Stock System Fe2O3 Iron (III) Oxide +6 -6 +3 Fe2 =0 -2 O3 + 1. Arrhenius acid yields an H ion as the only positive ion in the solution therefore formulas that contain hydrogen as the first elements are acids Binary Table E HF – Hydrofluoric HCl – Hydrochloric “Ate” “ic” “Ite” “ous” HBr – Hydrobromic HI – Hydriodic acid Types of Chemical Reactions 1. Synthesis = the formation of one product (right) from two or more reactants (left) 2H2+O2 2H2O 2. Decomposition = the breaking down of one reactant (left) to form 2 or more products (right) 2H2O 2H2+O2 3. Single Replacement = element1 + compound1 element2 + Compound2 In order for a single replacement reaction to occur the element must be more active (higher on table E) then the metal in the compound Li+NaCl Na + LiCl 4. Double Replacement = compound1 + compound2 compound3 + compound4 NaCl + KBr KCL + NaBr Balancing Equations In Chemistry there is a conservation of 3 things 1. MASS 2. CHARGE 3. ENERGY Use coefficients (big number in front) to balance equations. Coefficients represent the number of moles 2H2+1 O2 2H2O When balancing equations you must use the simplest whole number coefficients in your final answer. 2NH3 1N2+3H2 2:1:3 Sum of coefficients is 6