log pH H ⌈ ⌉ = - ⌊ ⌋ ⌈ ⌉ ⌊ 7.79 log H
advertisement

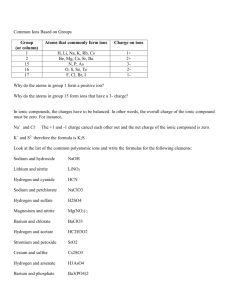
To simplify the measurement of acidity or basicity of a solution, the pH (hydrogen potential) measure was developed. Like the measurement of sound (decibels) or earthquakes (Richter scale), pH is a logarithmic scale. This means that numbers on the scale are related to the logarithm of some other quantity. In the case of pH, the measurement is given by pH log H where H is the concentration of hydrogen ions in moles per liter in the solution. Using this scale we can find the pH of any solution for which we know the concentration of hydrogen ions. For instance, the concentration of hydrogen ions in beer is 0.0000631 moles per liter. To find the pH of beer, write pH log 0.0000631 4.2 We can also take the pH of a solution and find the corresponding concentration of hydrogen ions. The pH of eggs is 7.79. By solving 7.79 log H for H , we can calculate the concentration. Isolate the logarithm by dividing both sides by ‐1, 7.79 log H Now convert to exponential form, H 107.79 Simplifying the right hand side yields H 0.0000000162 This is the concentration in moles per liter of hydrogen ions for eggs.