NAME________________________________ BIOLOGY 2401
advertisement

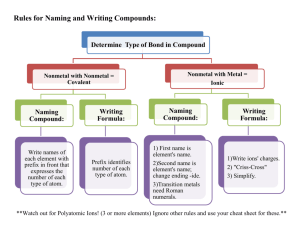
NAME___________ BIOLOGY 2401 DATE____________ INORGANIC CHEMISTRY FALL 2011 A 1. Write the symbol of the below elements. a. Sodium d. Phosphorus g. hydrogen - b. Chlorine e. oxygen h. iron - c. Potassium f. calcium – i. magnesium - 2. Define the below terms. a. electrolytes– b. free radical – c. radioisotope 3. A substance that dissociates in water and releases hydrogen ions is known as a/an acid/base and will cause the pH to go up/down. Substances that slow pH changes by donating or accepting hydrogen ions to change strong acids or bases into weaker acids or bases is known as a _________. 4. A change of pH from 7 to 9 would result in _____ times fewer hydrogen ions. This would make the solution more acidic/basic. A change of pH from 7 to 3 would result in ____ times more hydrogen ions. This would make the solution more acidic/basic. 5. Breathing slower than normal would cause the CO2 to increase/decrease and would result in hypercapnia/hypocapnia. This would result in the formation of more/less carbonic acid. This would result in a rise/lowering of pH and would result in acidosis/alkalosis. 6. Two atoms with the same atomic number but different atomic masses are known as __________________. An atom that has an unequal number of electrons and protons and that carries a charge is known as an _________. 7. A bond formed between atoms in which there is a sharing of electrons is known as a covalent/hydrogen/ionic bond. A positively charged ion is known as a cation/anion. Cl- is an example of a cation/anion. 8. Substances held together by ionic/covalent bonds dissolve in water. A reaction in which electrons are lost by a molecule is known as a oxidation/reduction reaction. 9. In a solution the substance that something is dissolved in is called the solute/solvent while the substance dissolved in another is called the solute/solvent. 10. List three functions for ions in the body. Bonus. Answer the below questions. a. List the three buffer systems observed in the body fluids in the acid, base, and buffer lab. b. Hypoventilation will increase/decrease the PCO2 in the blood and will cause the blood pH to increase/decrease. A decrease above normal of PCO2 is known as hypercapnia/hypocapnia. c. The most abundant cation found in the extracellular fluid would be ______________ while the most abundant anion found in the intracellular fluid would be __________.