ions sheet
advertisement
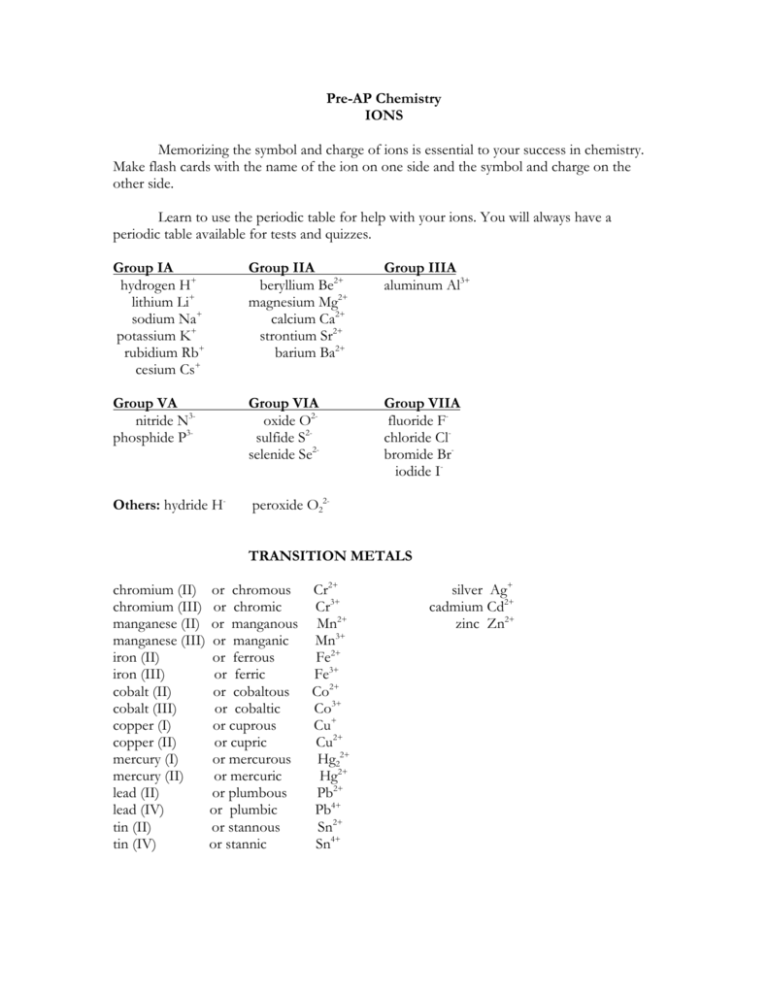
Pre-AP Chemistry IONS Memorizing the symbol and charge of ions is essential to your success in chemistry. Make flash cards with the name of the ion on one side and the symbol and charge on the other side. Learn to use the periodic table for help with your ions. You will always have a periodic table available for tests and quizzes. Group IA hydrogen H+ lithium Li+ sodium Na+ potassium K+ rubidium Rb+ cesium Cs+ Group IIA beryllium Be2+ magnesium Mg2+ calcium Ca2+ strontium Sr2+ barium Ba2+ Group IIIA aluminum Al3+ Group VA nitride N3phosphide P3- Group VIA oxide O2sulfide S2selenide Se2- Group VIIA fluoride Fchloride Clbromide Briodide I- Others: hydride H- peroxide O22TRANSITION METALS chromium (II) or chromous Cr2+ chromium (III) or chromic Cr3+ manganese (II) or manganous Mn2+ manganese (III) or manganic Mn3+ iron (II) or ferrous Fe2+ iron (III) or ferric Fe3+ cobalt (II) or cobaltous Co2+ cobalt (III) or cobaltic Co3+ copper (I) or cuprous Cu+ copper (II) or cupric Cu2+ mercury (I) or mercurous Hg22+ mercury (II) or mercuric Hg2+ lead (II) or plumbous Pb2+ lead (IV) or plumbic Pb4+ tin (II) or stannous Sn2+ tin (IV) or stannic Sn4+ silver Ag+ cadmium Cd2+ zinc Zn2+ POLYATOMIC IONS -1 charge dihydrogen phosphate H2PO4acetate C2H3O2hydrogen sulfate HSO4(bisulfate) hydrogen sulfite HSO3(bisulfite) hydrogen carbonate HCO3(bicarbonate) nitrite NO2nitrate NO3cyanide CNhydroxide OHpermanganate MnO4hypochlorite ClOchlorite ClO2chlorate ClO3perchlorate ClO4+1 charge ammonium NH4+ -2 charge hydrogen phosphate HPO42oxalate C2O42sulfite SO32sulfate SO42carbonate CO32chromate CrO42dichromate Cr2O72silicate SiO32-3 charge phosphate PO43phosphite PO33-











