Chemistry-Chapter-9-Assessment-Answers
advertisement

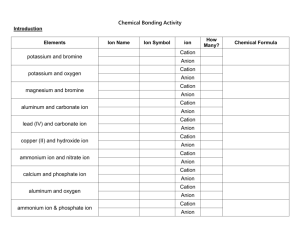
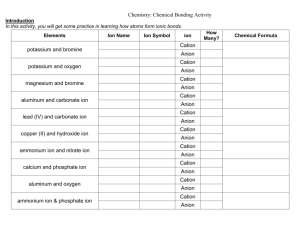
Chemistry – Chapter 9 Assessment Answers 9.1 Assessment 3. The positive charge of a group A metal is equal to its group number. The charge of a group A nonmetal is the group number minus 8. 4. from the number of electrons lost 5. –ite or –ate 6. Group 1A metals, 1+; Group 3A (aluminum), 3+’ Group 5A nonmetals, 37. A monoatomic anion is a single atom with a negative charge; a polyatomic anion is two or more bound atoms with a negative charge. 8. a.) K+, cation, potassium ion b.) O2- anion, oxide ion c.) Sn2+ cation, tin (II) d.)Br- anion, bromide ion e.) Be2+ cation beryllium ion f.) Co3+ cation, cobalt(III) ion 9. a.) NH4+ b.) Sn2+ c.) CrO42d.) NO3 9.2 Assessment 14. Write the name of the cation followed by the name of the anion. 15. Write the symbol of the cation followed by the symbol of the anion, then use subscripts to balance the charges. 16. Write the symbol for the metal ion followed by the formula of the polyatomic ion and balance the charges. Name the cation first followed by the anion. 17. a.) BeCl2 b.) Cs2S c.) Nal d.) SrO 18. a.) Cr(NO2)3 b.) NaClO4 c.) Mg(HCO3)2 d.) Ca(C2H3O2)2 19. a.) incorrect; charges are not balanced MgSO4 b.) c.) incorrect; charges are not balanced BeCl2 9.3 Assesment 20. Prefixes indicate the number of atoms of each element in a molecule of the compound. Chemistry – Chapter 9 Assessment Answers 21. Write the symbols for each element with a subscript corresponding to the prefix before each element in the name. 22. a.) nitrogen trichloride b.) boron trichloride c.) nitrogen triiodide d.) sulfur trioxide e.) dinitrogen tetrahydride 23. a.) carbon disulfide b.) CBr4 c.) dichlorine heptoxide d.) P2O3 24. a.) PCl5 b.) IF7 c.) CIF3 d.) IO2 25. No; the correct name is silicon tetrachloride. 9.4 Section Assessment 26. See the rules for naming on the SE facing page. (272) 27. The rules for writing the names of acids are used in reverse to write the formulas for acids. 28. The name of the cation is followed by the name of the anion. 29. a.) nitrous acid b.) permanganic acid c.) hydrocyanic acid d.) hydrosulfuric acid 30. a.) lithium hydroxide b.) lead(II) c.) magnesium hydroxide d.) aluminum hydroxide 31. a.) base b.) acid c.) base d.) base 32. a.) H2CO3 b.)H2SO3 c.) Fe(OH)3 d.) Sr(OH)2 Chemistry – Chapter 9 Assessment Answers 33. Acid, hydrogen; base, hydroxide ion 9.5 Assessment 35. Law of definite proportions and law of multiple proportions 36. Follow the arrows and answer the questions on the flowchart. 37. See the four guidelines on the facing SE page (page 278) 38. No; 2:1 39. a. Calcium carbonate b. lead (III) chromate c. tin(II) dichromate 40. a. Sn(OH)2 b. BaF2 41. a. Incorrect; a Roman numeral is not used with a Group A metal, calcium oxide b. correct c. correct d. Incorrect; ammonium ion, a polyatomic cation will not form a compound with a monatomic cation.